Author: Dr. Brandon Barnett
Case submitted courtesy of Dr. Claudia Garces
Case History: A 49 year old male presented for evaluation and treatment of teeth #19 and #20 with the following chief complaint “My dentist said I have an infection, but it doesn’t hurt.”
Medical history: Non-contributory, ASA I
Medications: None
Allergies: NKDA
HPI: #19 RCT completed in 2020 and #20 RCT completed in 2009 both with a prior dentist. Pt reports a sinus tract of about 3 weeks duration which has partially resolved after taking Augmentin 875-125 BID for an unspecified duration.
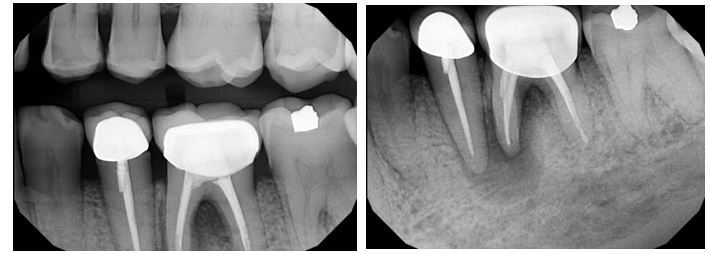
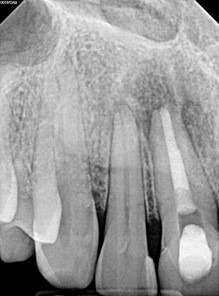
Radiographic evaluation: Periapical and bitewing radiographs of the mandibular left posterior quadrant show existing PFM crowns and prior root canal therapy on teeth #20 and #19, with a small, nonretentive post on #20. A large periapical radiolucency is noted at the M root of #19 which is confluent with a secondary large radiolucency at #20. The radiolucency on the M root of #19 extends towards the furcation.
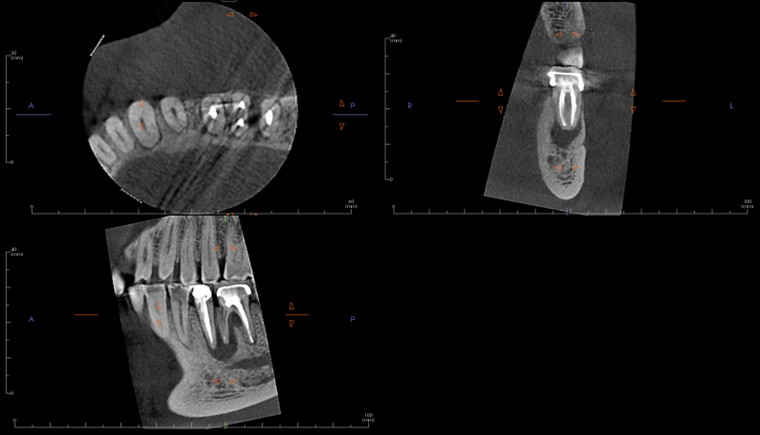
A limited FOV CBCT was exposed revealing an area of low density encompassing the root apices of #19 and #20. There was no evidence of missed/non-negotiated secondary anatomy. Existing root fills are greater than 0.5mm from the radiographic apex. The areas of reduced radiographic density are consistent with the appearance of apical periodontitis, and are not in close proximity to the inferior alveolar nerve.
Clinical evaluation: Probing depths ranged between 2-3mm in the mandibular left posterior quadrant save for the MB of tooth #19 where a probing depth of 10mm was noted. Sinus tract on the attached gingiva buccal to #19 was noted. Teeth #20 and #19 have class 1 mobility. Both PFM crowns on #19 and #20 present with clinically intact margins.
Testing:
#18 cold (+), percussion (-), palpation (-)
#19 cold (-), percussion (-), palpation (-)
#20 cold (-), percussion (-), palpation (-)
Diagnoses:
Tooth #20 Previously Treated, Asymptomatic Apical Periodontitis
Tooth #19 Previously Treated, Chronic Apical Abscess.
And now let's review the results from January's Case Challenge!:
Poll Results:
What would be your primary treatment choice?
- Non-surgical retreatment of #8 24%
- Apical surgery #8 53%
- Decompression 12%
- Non-surgical retreatment #8 with immediate apical surgery
- Non-surgical retreatment of #8 with immediate decompression 6%
- Intentional replantation #8
- Extraction #8 with replacement
- Extraction #7 and #8 with replacement 6%
- No treatment and monitor
Treatment Rendered: Sometimes we have the opportunity to combine multiple treatment strategies in complex cases. In this case, non-surgical retreatment of #8 was performed in tandem with decompression. After discussing his treatment plan with the referring GD, the patient was seen first by his GD for removal of the existing crown and placement of a long-term milled temporary crown. The patient then returned to our office for the post and gutta-percha removal with placement of intracanal calcium hydroxide. This was followed by immediate decompression of the lesion. The decompression technique used was the simultaneous needle aspiration and irrigation method described by Hoen et al. (JOE, 1990). This involves placement of two 16 gauge needles into the lesion with simultaneous aspiration and irrigation with saline (no long-term drain was used). 30ml of saline was used to irrigate the lesion until the aspirated fluid was clear.
After one month, the patient returned for follow-up and the buccal sinus was still present. The tooth was re-accessed and the calcium hydroxide was replaced. At the two month mark, the buccal sinus tract was healed, but the canal still had continued mild drainage internally. A third round of calcium hydroxide was applied and left in place for 3 months. When the patient returned (5 months after initiating treatment), evidence of healing was observed radiographically around #7 and #8. RCT #8 was completed with an MTA obturation and RMGI orifice barrier. Sufficient ferrule and peripheral tooth structure was present and another post was considered not necessary.
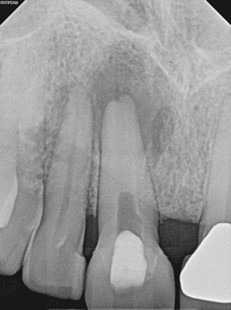
After 2 months of calcium hydroxide. Early evidence of periapical healing, The buccal sinus tract healed but the canal was still draining internally. PA taken immediately after third round of calcium hydroxide (powder) applied.
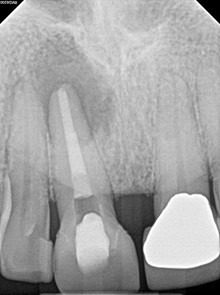
Immediate Post-op (after long-term CaOH for 5 months)
6-month recall: Tooth #8 was asymptomatic and with no buccal sinus tract present. Tooth #7 remains responsive to cold testing. The patient had not returned to their GD for the permanent crown. The mobility in #8 was notably reduced and significant healing was observed radiographically. The lesion may heal with an apical scar, but apical surgery will likely not be necessary. The patient was scheduled for a 12-month recall and advised to return to their GD soon for the permanent crown.
By Dr. Adam Gluskin
Some of my earliest childhood memories include conversations about endodontics at the dinner table. My dad, a full-time professor of endodontics, would spend his evenings refining lectures, studying radiographs, and drafting manuscripts at the end of a long workday. Long before I understood anything about the mechanics of root canal treatment or the impact it could have on a patient’s life, I understood that this specialty mattered deeply to him.
Choosing endodontics for myself brought new depth to a familiar path. Endodontics demands precision, patience, and humility. My father often reflects back on the humility of his greatest mentor, Dr. Sam Seltzer, who would often say “you could fill 20 libraries with what we don’t know”. Dr. Seltzer was not only his program director, but a pioneer in our fundamental understanding of endodontics. Even as technology advances at remarkable speed, we are still guided by the biologic principles that Dr. Seltzer and his contemporaries laid down for us.
Sharing this profession with my father has been one of the great privileges of my life. We represent different generations of the same specialty. He trained well before microscopes were commonplace; I trained never knowing a world without them. He adapted to the rise of nickel-titanium instrumentation; I entered a field already transformed by it. Today we practice with CBCT-guided diagnostics, advanced irrigation, microscopic precision, and expanding regenerative concepts. What once seemed aspirational is now routine. My conversations with my father often reflect how far endodontics has come, and how much its core principles remain unchanged.
We also both agree that despite these advancements, the essence of endodontics is still profoundly human. It is relieving pain. It is earning trust. It is connecting with patients in a moment when they feel most vulnerable.
For those entering the field without a family connection to endodontics, I want to emphasize something equally important: mentorship is core to this specialty. I have had the unique privilege of learning alongside my father, but every endodontist has access to a professional family. Our mentors, co-residents, program directors, and colleagues become the support system that carries us through challenging cases and pivotal career decisions.
Lean into that community. Call a former co-resident. Reach out to a mentor. Attend professional meetings. Ask a lot of questions. Endodontics rewards curiosity and humility, and no one succeeds in isolation.
As residents and new practitioners, we are starting our careers at an exciting moment in the evolution of our field. The pace of endodontic innovation feels extraordinary right now. But the most important constant is not the technology, but the commitment to excellence that defines our specialty. I am grateful not only for the opportunity to practice endodontics, but for the legacy, mentorship, and community that shape it. It is a specialty built on progress and sustained by those willing to continually learn and teach.
For those just beginning this journey: lean in and engage. The future of endodontics is bright, and you are an essential part of it.
 By Priscilla L. Carpenter Lockhart, DDS, MS
By Priscilla L. Carpenter Lockhart, DDS, MS
February has a way of making us a little sentimental. Hearts, roses, sweet treats; and if you’re anything like me, maybe just a faint hint of sodium hypochlorite in the air. (Romantic, I know.)
But jokes aside, this time of year is actually a great reminder of something we don’t always pause to say: we really love endodontics.
A Love Letter to Endo
What is it about this specialty that pulls us in and keeps us here?
Maybe it’s the quiet satisfaction of finding those elusive canals.
Maybe it’s the moment a patient who walked in miserable leaves relieved.
Maybe it’s the precision, the problem-solving, the constant evolution of our field.
Endodontics is equal parts art, science, and patience, and let’s be honest, just enough challenge to keep us humble. We’ve all had those cases that test our skills (and our emotional stability), but that’s part of the love story too. The growth. The grit. The wins that feel really earned.
Sharing the Endo Love
Love for this specialty doesn’t just live in our operatories, it grows when we share it.
- Mentor a student who’s endo-curious.
- Teach a referring doctor something new.
- Encourage a co-resident before boards.
- Share a clinical pearl that makes someone’s day easier.
- Show patients what modern endodontics really looks like.
Passion is contagious. Knowledge multiplies. And our specialty gets stronger every time we lift someone else up.
Mark Your Calendars ❤️📅
MTA March Madness
Be on the lookout for more information as we gear up for some good basketball and friendly competition! There’s a nice prize at the end of this rainbow.
AAE Foundation Freedom Scholarship – Due March 6, 2026
The AAE Foundation Freedom Scholarship supports residents pursuing advanced endodontic education, with three residents selected to receive this prestigious award. If you or someone you know may be eligible, now is the time to apply and spread the word.
ABE Oral Examination – March 6–7, 2026
St. Louis, MO
To everyone preparing: we see the work you’ve put in. Stay focused, you’ve got this!
AAE Annual Meeting – April 15–18, 2026
Salt Lake City, UT
One of the best times of the year to reconnect, recharge, and re-energize your love for endodontics. Start making your plans now!
APICES – August 14–15, 2026
St. Louis, MO
It may feel far away, but it will be here before we know it. Stay tuned for more details on this can’t-miss event.
As we move through February, I hope you take a moment to reflect on what first made you fall for this specialty, and maybe find a small way to share that enthusiasm with someone else.
Because whether it’s love in the air… or just NaOCl… one thing is certain:
Endodontics still has our hearts.
With Love (and properly irrigated canals),
Priscilla Carpenter Lockhart, DDS, MS, is Chair, Resident and New Practitioners Committee and Diplomate, American Board of Endodontics.
 By John A. Mitsos, CLU®, CLTC
By John A. Mitsos, CLU®, CLTC
Dentists and dental specialists turn to search engines for more than clinical questions. They also look for answers about financial security. Phrases like “disability insurance for dentists,” “own-occupation disability,” and “what happens if I can’t practice dentistry” appear frequently in financial and dental-planning content because they reflect real professional concerns.
What those searches reveal is something fundamental: the risk of losing the ability to earn a professional income. For dentists, that risk is unusually concentrated. If injury, illness, or neurological changes affect clinical work, income often stops with it.
The real risk isn’t rare, it’s routine!
When people think about disability, they often picture traumatic accidents. In dentistry, the dominant risks are far less dramatic and far more common. Musculoskeletal disorders, stress-related conditions, autoimmune disorders, and cardiovascular events represent sources of long-term disability among healthcare professionals.
Across the general working population, roughly one in four people will experience a disability long enough to interrupt their career before retirement age. That probability is high enough to be treated as a planning assumption rather than a remote possibility, particularly in a profession as physically demanding as dentistry.
Why dentists are financially exposed
Dentists’ income is not only high; it is directly tied to physical capability and technical precision. If that capacity is reduced, income declines immediately.
Unlike many corporate professionals, dentists typically cannot pivot into a new role that pays anything close to chairside earnings. Even part-time or limited clinical work often results in a substantial income drop. Meanwhile, financial obligations such as student or practice loans, payroll, and family and lifestyle expenses do not stop.
Without disability income insurance, a prolonged illness or injury can become a liquidity crisis far faster than expected. Even well-funded savings and investment portfolios can erode quickly when high fixed expenses collide with reduced income. Losing the ability to practice dentistry is not just a health issue, it can be a career-defining financial event.
Social Security is not a dentist’s safety net
Social Security Disability Insurance (SSDI) pays benefits only if you are unable to work in any occupation, not just dentistry. If you could theoretically earn income in another field—teaching, consulting, or administrative work—you may be denied. Approval rates are low, the process is slow, and benefit amounts are modest relative to a dentist’s earnings. From a planning standpoint, SSDI is not income replacement; it is a last-resort public program.
Why “own-occupation” coverage matters
Dentists do not search for just any disability policy: they search for own-occupation coverage. That distinction is critical.
A true own-occupation policy pays benefits if you cannot perform the duties of dentistry, even if you could earn income in another profession. Without it, a policy may deny a claim if you are capable of doing non-clinical work, regardless of the income gap.
Dentists should also consider:
- Residual or partial disability benefits, which pay when work capacity is reduced
- Future purchase options, which allow coverage to increase as income grows
- Cost-of-living adjustments, which help preserve benefit value during long claims
These features determine whether a policy truly protects earning power or simply appears adequate on paper.
Practice owners face an added layer of risk
For practice owners, a prolonged absence due to disability can destabilize the business. Staff salaries, rent, equipment leases, and loan obligations continue regardless of clinical availability. Business overhead expense (BOE) disability insurance can help keep a practice operating, but it does not replace personal income. Dentists who own practices typically need both forms of coverage to protect their professional and personal financial structures.
Cost versus consequence
Disability income insurance is typically a small fraction of a dentist’s income, depending on age, health, and policy design. That cost is tiny relative to the exposure it protects.
One long-term disability claim can represent millions of dollars in lost lifetime earnings. Few financial decisions offer a higher return on risk reduction.
The fact that so many dentists actively search for this coverage tells an important story: income risk feels more real than most people admit. Disability income insurance does not eliminate uncertainty, but it converts fear into something manageable. For a profession built on precision, that stability is worth protecting.
About Treloar & Heisel
Treloar & Heisel, an EPIC Company, is a premier financial services provider to dental and medical professionals across the country. We assist thousands of clients from residency to practice and through retirement with a comprehensive suite of financial services, custom-tailored advice, and a strong national network focused on delivering the highest level of service. Insurance products offered through Treloar & Heisel, LLC.
For more information, visit us at www.treloaronline.com.
TH-26-004
source: https://www.ssa.gov/oact/NOTES/ran6/an2024-6.pdf?utm_source=chatgpt.com
John A. Mitsos, CLU®, CLTC, is Financial Services Professional and Training Specialist, Treloar & Heisel, LLC. He can be reached at jmitsos@treloaronline.com.
The American Association of Endodontists (AAE) continues to advocate for fair and transparent insurance practices that support patient access to care and the sustainability of specialty practices. As part of this effort, AAE recently submitted comments in support of Massachusetts Senate Bill 704, legislation addressing the use of virtual credit cards by dental insurance providers.
Virtual credit card payments often impose processing fees that reduce reimbursement for care already delivered, creating unnecessary administrative costs for dental practices. In its comments to Massachusetts lawmakers, AAE emphasized that these practices can strain provider resources and discourage participation in insurance networks—ultimately limiting patient access to care.
AAE expressed strong support for provisions in S.704 that would prevent insurers from mandating credit cards as the sole method of reimbursement and require affirmative provider consent before credit card or virtual credit card payments are initiated. The legislation also promotes greater transparency by ensuring providers receive clear remittance information and advance disclosure of any associated fees.
Endodontists frequently provide urgent, specialized treatment for patients experiencing dental infections and pain. AAE noted that excessive administrative burdens and hidden payment fees can interfere with the timely delivery of this care. By allowing providers to choose their preferred payment method, S.704 helps protect practice sustainability while supporting efficient, patient-centered care.
AAE’s engagement on S.704 reflects its broader commitment to advocating for policies that reduce unnecessary administrative barriers, promote fairness in reimbursement, and strengthen the dental community. By supporting thoughtful reforms in Massachusetts, AAE continues to stand up for endodontists and the patients who rely on their specialized care.
The American Association of Endodontists (AAE) continues its strong advocacy for policies that support patient access to timely, high-quality care and protect the sustainability of specialty practices. As part of this effort, AAE recently submitted formal comments supporting Florida Senate Bill 1130, legislation aimed at improving insurance claims payment practices for health care providers.
SB 1130 addresses insurer practices that can undermine patient care, including inappropriate downcoding and delayed or unclear reimbursement decisions. In its comments to Florida lawmakers, AAE emphasized that these practices create unnecessary administrative burdens for providers and can interfere with patients’ ability to receive prompt, medically necessary treatment.
Endodontists routinely deliver urgent care for patients experiencing severe dental pain and infection—conditions that often require immediate intervention to prevent serious complications. AAE highlighted that SB 1130 would help preserve access to this care by establishing clearer restrictions on downcoding, increasing transparency when payment reductions occur, and strengthening prompt payment standards.
The legislation also promotes greater accountability in prior authorization and utilization review processes, including improved electronic systems and clearer standards for insurer decision-making. These reforms are designed to reduce treatment delays, enhance continuity of care, and ensure reimbursement aligns with the services provided.
AAE’s support for SB 1130 reflects its broader commitment to advocating for fair, transparent insurance practices that allow endodontists to focus on patient care rather than administrative obstacles. By engaging early with policymakers, AAE continues to champion legislation that strengthens specialty practices and protects access to essential dental care.
Following its strong opposition to earlier proposals that threatened to weaken specialty advertising standards in Wisconsin, the American Association of Endodontists (AAE) welcomed recent action by the Wisconsin Dentistry Examining Board to clarify and strengthen its dental specialty advertising rules. The Board’s proposed revisions to Chapter DE 6 represent a meaningful step toward protecting patients from misleading claims while reinforcing the value of accredited specialty education.
In formal comments submitted to the Board, the AAE expressed support for provisions that clearly define what constitutes false, misleading, or deceptive advertising. By providing greater specificity and transparency, the proposed rules help ensure consistency in enforcement and offer clearer guidance to dentists seeking to comply with advertising requirements. Most importantly, these safeguards enhance consumer protection and support informed decision-making by patients.
The AAE also commended the Board for reinforcing the principle that the title “specialist” must be reserved for dentists who have completed a postdoctoral educational training program accredited by the Commission on Dental Accreditation (CODA). Requiring dentists who are not specialists to clearly identify themselves as general dentists is a critical protection against patient confusion and aligns with public expectations regarding specialty credentials.
At the same time, the Association raised concerns about the continued use of alternative titles—such as “implantologist”—that may function as a proxy for specialty designation. While the proposed rules require disclaimers identifying these providers as general dentists, the AAE cautioned that such distinctions may not be readily understood by patients. From a consumer perspective, alternate terminology can still imply a level of specialty expertise that does not reflect CODA-accredited training. The AAE encouraged the Board to further strengthen the rule by limiting advertising titles to prevent unintended confusion.
Finally, the AAE strongly supported provisions preventing a dentist from implying that all practitioners within a group practice are specialists unless each individual has earned that designation. This clarification is essential to preserving the integrity of specialty recognition and ensuring that patients receive accurate information when choosing their provider.
Together, these proposed revisions reflect a shared commitment to truth in advertising, patient protection, and professional accountability. The AAE’s engagement in Wisconsin underscores its continued advocacy for clear, enforceable standards that uphold the value of accredited specialty education and protect the public trust. By supporting thoughtful regulatory improvements, the Association continues to safeguard your ability to practice as a recognized specialist.
As the 2026 legislative cycle kicks off, the American Association of Endodontists (AAE) is already actively engaging with policymakers to strengthen the dental community and expand access to high-quality oral health care. AAE recently submitted a series of comment letters supporting state legislation to ratify the Dentist and Dental Hygienist Interstate Compact, underscoring the Association’s continued commitment to effective, collaborative advocacy.
To date, AAE has expressed support for compact legislation introduced in Arizona, Missouri, New Jersey, New Hampshire, New Mexico and Oklahoma. These early advocacy efforts highlight AAE’s proactive approach to shaping policy that supports a strong, mobile dental workforce and benefits patients across the country.
In its communications with lawmakers, AAE emphasized that interstate licensure compacts help address workforce shortages—particularly in rural and underserved areas—while maintaining rigorous licensure and public safety standards. By reducing unnecessary administrative barriers, the compact allows qualified dentists and dental hygienists to practice where they are needed most, strengthening collaboration and continuity of care throughout the dental community.
AAE also noted that the compact promotes accountability and public trust through background checks, coordinated disciplinary data sharing, and consistent licensure requirements across participating states. These safeguards ensure that workforce flexibility enhances, rather than compromises, patient protection.
As the specialty organization representing more than 8,000 endodontists worldwide, AAE continues to advocate for policies that recognize the essential role of dental specialists in preserving natural teeth, managing dental pain, and supporting comprehensive oral health care. Supporting interstate compact legislation aligns with AAE’s broader mission to strengthen the dental workforce and the dental community as a whole.
AAE will continue to closely monitor additional interstate compact bills introduced during the 2026 legislative cycle and will support legislation that advances patient access, protects public safety, and strengthens the dental profession. Through sustained engagement with lawmakers and coalition partners, AAE remains committed to advancing policies that benefit endodontists, the broader dental community, and the patients they serve.
The Minnesota Board of Dentistry is considering proposed revisions to state regulations that would expand the scope of practice for dental therapists. These changes represent a significant shift in Minnesota’s long-standing regulatory framework governing the delivery of dental care. If adopted, the proposal would blur established professional roles, weaken accountability, and raise serious concerns about patient safety and standards of care.
In response, the American Association of Endodontists (AAE) submitted formal opposition to the Board, emphasizing that scope-of-practice decisions must align with a provider’s education, training, and level of clinical responsibility. Endodontists complete rigorous, Commission on Dental Accreditation (CODA)–accredited postdoctoral education to ensure competence in diagnosing and managing complex disease. Expanding dental therapists’ authority beyond their intended supportive role risks delegating critical clinical responsibilities to practitioners who are not trained to assume them independently.
The AAE also underscored that this issue extends beyond access to care. While improving access is an important policy objective, it cannot come at the expense of safety, quality, or professional standards. Expanding scope of practice by redefining core clinical responsibilities sets a dangerous precedent and undermines the dentist-led model of care that has long protected patients. Dental therapists play an important role on the oral health care team, but that role is designed to complement, not replace, dentist oversight and accountability.
Additionally, expanding scope of practice risks creating confusion for patients about provider qualifications and responsibility for care. When regulatory lines are blurred, patients may reasonably assume that all providers are equally trained to deliver the same level of care. This confusion erodes public trust in the dental care delivery system and conflicts with the Board’s fundamental responsibility to protect public health.
The AAE urged the Minnesota Board of Dentistry to reject the proposed scope-of-practice expansion and to maintain existing regulatory definitions that appropriately reflect education, training, and professional responsibility. The Association also called for additional stakeholder engagement, including meaningful dialogue with dental specialty organizations, before advancing any further changes.
Through consistent, evidence-based advocacy, the AAE continues to stand firm against regulatory efforts that threaten patient safety and professional standards. Our engagement in Minnesota reflects the Association’s ongoing commitment to protecting the integrity of dental specialties—and to supporting endodontists as they provide high-quality, specialist-level care to patients every day.

 Endodontics is hard work, both physically and mentally. I’m not complaining, just stating facts. Anyone who has spent some time in the “trenches” practicing clinical endodontics can attest. The other evening as I was pondering my day, I felt especially tired. The reality was I didn’t really do anything different than the previous days. What was different was I had a number of patients questioning the scientific validity of what we do. We’ve all heard it: “Root canals cause cancer, root canals lead to other diseases, how can you leave a dead organ in the body”. My problem, I realized was not that I was any more physically tired than the day before, I was however mentally taxed! As clinicians, we’ve always had to correct patient misunderstandings, misconceptions and mistruths but over the past decade and especially the last couple of years we’ve entered a new era, one in which misinformation isn’t merely occasional, it’s systemic. What patients see on TikTok, hear from influencers, or read in online forums now competes directly with our training and expertise. Instagram is the new Dr. Google where once upon a time we were “bothered”, occasionally, by a patient that looked something up online. The consequences are no longer limited to chair-side confusion-they affect treatment decisions, access to care, public trust, and even governmental policy.
Endodontics is hard work, both physically and mentally. I’m not complaining, just stating facts. Anyone who has spent some time in the “trenches” practicing clinical endodontics can attest. The other evening as I was pondering my day, I felt especially tired. The reality was I didn’t really do anything different than the previous days. What was different was I had a number of patients questioning the scientific validity of what we do. We’ve all heard it: “Root canals cause cancer, root canals lead to other diseases, how can you leave a dead organ in the body”. My problem, I realized was not that I was any more physically tired than the day before, I was however mentally taxed! As clinicians, we’ve always had to correct patient misunderstandings, misconceptions and mistruths but over the past decade and especially the last couple of years we’ve entered a new era, one in which misinformation isn’t merely occasional, it’s systemic. What patients see on TikTok, hear from influencers, or read in online forums now competes directly with our training and expertise. Instagram is the new Dr. Google where once upon a time we were “bothered”, occasionally, by a patient that looked something up online. The consequences are no longer limited to chair-side confusion-they affect treatment decisions, access to care, public trust, and even governmental policy.