Cell-Based PDL Regeneration for the Management of Avulsed Teeth

By Dr. Hacer Aksel
Despite the numerous technological advancements and improvements in endodontic treatments, avulsion remains a significant challenge for dental professionals due to the complex nature of the injury, the limited time for intervention, and the potential for unfavorable outcomes.
Dental avulsion refers to the complete displacement of a tooth from its alveolar socket that causes pulp necrosis and destruction of periodontal tissues. It accounts for up to 16% of dental injuries and mostly occurs in children. Pulp necrosis can be treated without difficulty, but damage to the periodontal ligament (PDL) is challenging and causes replacement resorption and tooth loss. The replantation time is critical, and the tooth needs to be replanted quickly (especially in the first 5 min) to prevent loss of the PDL. In this case, emergency personnel with the knowledge of the emergency protocol are needed at the injury site. If replantation is not possible at the site of the injury, the tooth must be stored in an appropriate medium until the patient can be seen at the dental office. Hanks’ balanced salt solution, milk, saliva, saline, or water has been used as storage media, while water is the least desirable. However, these solutions can be effective only if available at the time of injury.
According to current guidelines, the management of avulsion can be categorized into four stages based on extraoral time and storage conditions. Stage 1 represents onsite replantation in less than 5 min, the ideal treatment condition. Most PDL cells are vital. In stage 2, the tooth is stored in a wet condition for less than an hour, and there are still PDL cells, but the vitality might be reduced. In Stages 3 and 4, extra-oral time is more than an hour regardless of wet or dry storage, and PDL cells are most likely nonvital. In these stages, the prognosis is unfavorable due to the development of replacement resorption. Unfortunately, most avulsed teeth undergo delayed replantation with an average clinical delay of more than one hour, which results in replacement resorption (1). This resorption continues until the root is replaced by bone, leading to crown fracture and tooth loss (Fig.1). After it starts, replacement resorption progresses without additional inflammatory stimulation, and it can occur in 51% of avulsion cases (2).
Current treatment approaches aim to slow the osseous replacement and maintain the tooth for years by reducing the initial inflammation and making the root surface resistant to resorption.
Placement of tetracycline and corticosteroids as an intracanal medicament has been shown to inhibit surrounding inflammation (3). Removing nonvital PDL using sodium fluoride solution can also limit the initial inflammation and the extent of resorption, but in fact, no treatment is effective in preventing replacement resorption when it starts. The other option can be the regeneration of PDL by periodontal ligament stem cells (PDLSCs) that can potentially prevent or reduce the replacement resorption, especially in the case of extended extraoral time. The question is, “How can we regenerate PDL on the root surface with normal functions?” To understand this, we completed an extensive literature search and conducted some in vivo studies.
Cell-based PDL regeneration was initially used to address periodontal defects (4, 5). An approach involving a cell sheet technique was utilized to investigate the potential of PDLSCs in regenerating PDL and cementum on the root surface. The cell sheet, comprising a layer of cells, can be created using a temperature-responsive culture dish. Briefly, by lowering the temperature of the dish, the cell sheet can be harvested without causing damage to the cellular attachments and extracellular matrix (ECM), enabling cells to adhere to host tissues with minimal cell loss. Subsequently, PDL regeneration via the cell sheet technique was explored for avulsed teeth. We conducted experiments on de novo PDL regeneration in a mouse model. Human roots, after removal of cellular contents, were coated with a human PDLSC sheet, attached to a biodegradable membrane, and transplanted into the subcutaneous space of the mouse. Histological analysis showed the formation of a PDL-like tissue layer around the root and the regeneration of cementum on the root surface. Furthermore, a recent systematic review indicated an 80% occurrence of new PDL formation after the cell-based treatment of avulsion in vivo (6). While preliminary, these cell-based techniques suggest that avulsed teeth could potentially be managed through cell-based PDL regeneration.
A cell-free approach can also be used in some clinical conditions. The remaining PDL on the alveolar socket can preserve vitality and migration ability during the first week of the healing (7). An additional approach using platelet-rich fibrin (PRF) can improve the proliferation and migration ability of these remaining PDL cells (8). With the release of growth factors, the fibrin ultrastructure of PRF can support cell attachment, proliferation, and differentiation (9). In the absence of PRF, previous studies reported ankylosis due to a lack of PDL regeneration (8, 9).
Based on the existing literature and our prior investigations, we have expanded the stages for avulsion management into five distinct categories (Fig. 2). In Stage 1, immediate replantation is recommended. In Stage 2, if the extraoral time is under 1 hour and the tooth is kept wet, immediate replantation with PRF is advised to facilitate the migration, proliferation, and differentiation of remaining PDL cells. RCT needs to be started in mature teeth within 10 days. Stage 3 involves extraoral times exceeding 1 hour but less than 7 days with wet storage, where RCT, followed by replantation with PRF is recommended. The prognosis at this stage is less favorable than in Stage 2 due to relying on the remaining PDL cells in the tooth socket for regeneration. Stage 4 involves situations with extraoral times under 7 days, but dry storage, RCT, followed by cell-based PDL regeneration is the sole option due to the absence of vital PDL cells. In Stage 5, where the extraoral time spans weeks and regardless of the storage conditions, preparing the alveolar socket for replantation is essential. However, our recent findings highlight the lack of PDL regeneration with this approach, possibly due to inflammatory responses resulting from socket drilling (10).
The PDL cells can be derived from third molar teeth and cryopreserved for timely use (11). However, more standardized protocols are required, especially for extended extraoral times. Exploring the role of extracellular matrix proteins can be promising in guiding the cells to produce cementum-PDL-like tissues. In addition, 3D bioprinting technology can allow the seeding of multiple cell types and growth factors onto irregular root surfaces to restore the cementum-PDL-bone complex. PDL regeneration using immunomodulation is also required for replantation, especially when socket preparation is needed.
It is imperative to advance the treatment of avulsed teeth, particularly in delayed cases, using these innovative cell-mediated approaches. Without such progress, the management of avulsed teeth is likely to remain stagnant.
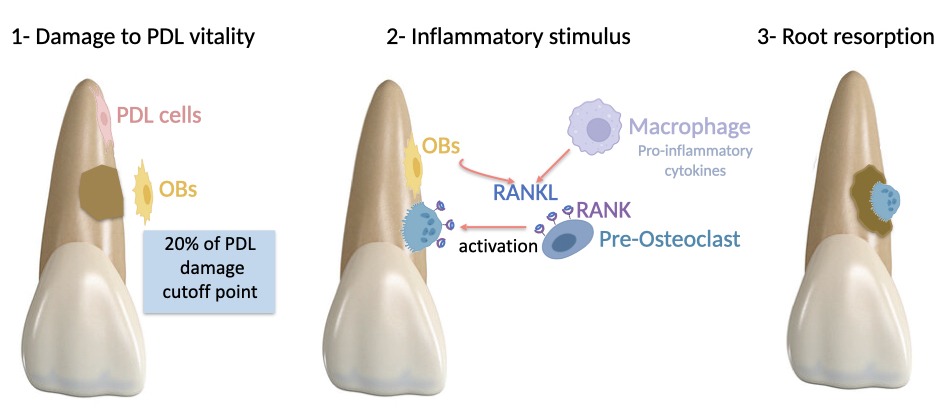
Figure 1
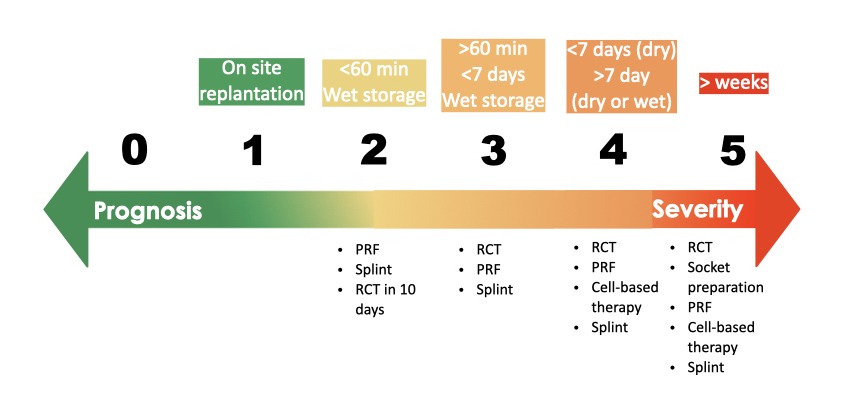
Figure 2
Figure Legends
Figure 1. The injury damages the PDL and results in the migration of the surrounding PDL and bone cells. If more than 20% of PDL is damaged, the damaged area is mostly repopulated by fast-moving bone precursor cells rather than slower-moving PDL cells, causing ankylosis and replacement root resorption. Osteoblasts (OBs) express RANKL as a protein, and osteoclasts carry the respective RANK receptor. Activation of immune cells by injury leads to the release of cytokines, that induces osteoblasts to secrete RANKL. The binding of RANKL to RANK activates osteoclasts, and osteoclasts start to resorb root (Created with BioRender).
Figure 2. Stages and proposed management of avulsion
References
- Müller DD, Bissinger R, Reymus M, Bücher K, Hickel R, Kühnisch J. Survival and complication analyses of avulsed and replanted permanent teeth. Sci Rep 2020;10:2841.
- Souza BDM, Dutra KL, Kuntze MM, et al. Incidence of Root Resorption after the Replantation of Avulsed Teeth: A Meta-analysis. J Endod 2018;44:1216-27.
- Chen H, Teixeira FB, Ritter AL, Levin L, Trope M. The effect of intracanal anti-inflammatory medicaments on external root resorption of replanted dog teeth after extended extra-oral dry time. Dent Traumatol 2008;24:74-8.
- Flores MG, Hasegawa M, Yamato M, Takagi R, Okano T, Ishikawa I. Cementum-periodontal ligament complex regeneration using the cell sheet technique. J Periodontal Res 2008;43:364-71.
- Iwata T, Yamato M, Tsuchioka H, et al. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials 2009;30:2716-23.
- Chew JRJ, Tan BL, Lu JX, Tong HJ, Duggal MS. Cell-based therapy for tooth replantation following avulsion: a systematic review. Tissue Eng Part B: Reviews 2022;28:351-63.
- Cardaropoli G, Araújo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol 2003;30:809-18.
- Ji B, Sheng L, Chen G, et al. The combination use of platelet-rich fibrin and treated dentin matrix for tooth root regeneration by cell homing. Tissue Eng Part A 2015;21:26-34.
- Zhao Y-H, Zhang M, Liu N-X, et al. The combined use of cell sheet fragments of periodontal ligament stem cells and platelet-rich fibrin granules for avulsed tooth reimplantation. Biomaterials 2013;34:5506-20.
- Aksel H, Zhu X, Gauthier P, Zhang W, Azim AA, Huang GT. A new direction in managing avulsed teeth: stem cell-based de novo PDL regeneration. Stem Cell Res Ther 2022;13:34.
- Kaku M, Kamada H, Kawata T, et al. Cryopreservation of periodontal ligament cells with magnetic field for tooth banking. Cryobiology 2010;61:73-8.
Dr. Hacer Aksel is Clinical Assistant Professor ,Division of Endodontics, University at Buffalo – School of Dental Medicine.




