Infection Control in Regenerative Endodontic Procedures
 By Su-Min Lee, D.D.S., M.S., DScD
By Su-Min Lee, D.D.S., M.S., DScD
The traditional treatment of immature teeth with necrotic pulps relied on apexification procedures involving long-term calcium hydroxide Ca(OH)2 treatment with multiple visits or on one-step apexification placing an apical plug of a mineral trioxide aggregate (MTA). Even though these treatments often result in the resolution of clinical signs and symptoms of apical periodontitis, they do not regenerate the physiology of the pulp–dentine complex, nor do they allow for further root growth. Thus, immature teeth remain with thin canal walls and poor root-crown ratios, increasing susceptibility to root fractures and lower long-term survival rates. A better alternative in such cases would be performing regenerative endodontic procedures (REPs). REPs involve removing diseased or necrotic pulp tissue and replacing it with healthy pulp tissues to revitalize teeth. This optimally translates to the complete restoration of pulpal function and subsequent maturation of root development and thus, may confer a better long-term prognosis. A growing body of evidence has showed the clinical feasibility of this approach, making it likely that REPs will become established procedures in the endodontic treatment spectrum. However, there is still variable predictability of root maturation, as well as evidences that the newly formed tissues may not present root regeneration of the native pulp-dentin complex, but some degree of wound healing or repair.
Induction of periapical bleeding into the canal space is a crucial step in REPs. Blood clots in the canal space could serve as a matrix or scaffold to promote pulp tissue wound healing. Provoked periapical bleeding brings in an influx of mesenchymal stem cells (MSCs) from the periapical area to the canal space. In addition, blood contains many platelet-derived growth factors. Therefore, induced periapical bleeding provides fibrin scaffold, MSCs, and bioactive growth factors, which are three key elements for pulp tissue engineering in the canal space. However, it should be emphasized that pulp revascularization from this biologically based approach is more favorable in a bacteria-free environment, which requires a clean and disinfected root canal system prior to cell colonization. Recently, there have been a few case reports showing the recurrence of apical lesion and/or a suboptimal result such as no additional root formation from REPs. Their histological analysis demonstrated that there were remaining bacteria in the root canal. Moreover, it is not known to what degree the root canal system needs to be disinfected in order for clinical success to be evident. Thus, many investigators in translational studies for REPs have focused their efforts on establishing the biological basis for clinical protocols that could achieve a balance between adequate disinfection and preservation of optimum regenerative potential of MSCs in periapical tissue.
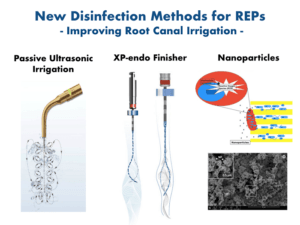
In the case of REPs for immature necrotic teeth, clinicians often face the challenge of adequately debriding large infected root canals. These canals are also compromised by fragile underdeveloped dentinal walls, which represent a contraindication for mechanical instrumentation. Thus, chemical debridement remains the primary form of disinfection in REPs. Sodium hypochlorite (NaOCl) is the most widely used agent for chemical debridement in endodontic procedures. However, NaOCl at its maximum clinically used concentration denatures crucial growth factors in the dentin and has a profoundly detrimental effect on stem cell survival. Canals need to be irrigated with 1.5% NaOCl (20 mL/canal, 5 min) with a side-vented irrigating needle positioned about 1 mm from the root end. In addition, passive ultrasonic irrigation (PUI) and using XP-endo finisher (FKG Dentaire SA, La Chaux-de-Fonds, Switzerland) could maximize its anti-microbial and -biofilm activity in these large canals. Subsequently, canals need to be irrigated with sterile physiological saline (5 mL) to minimize the cytotoxic effects of NaOCl on vital tissues. Furthermore, the application of 17% ethylenediaminetetraacetic acid (EDTA; 20 mL/canal, 5 min) as a final step in the irrigation protocol minimizes the detrimental effects of NaOCl. Moreover, 17% EDTA has a positive effect on the survival, differentiation, and attachment of stem cells. More importantly, EDTA promotes the release of biologically active growth factors embedded in the dentin matrix during dentinogenesis, which participate actively in regenerative processes such as angiogenesis and stem cell proliferation, migration, and differentiation. Thus, a final canal rinse with EDTA should be conducted prior to induction of periapical bleeding.
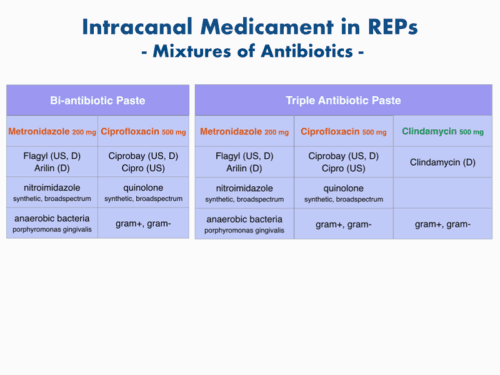
There is a particular combination of antibiotics that effectively disinfects root canal systems and increases revascularization of avulsed and necrotic teeth. This combination includes ciprofloxacin, metronidazol, and minocycline with a 1:1:1 ratio which is known as triple antibiotic paste (TAP). Due to its side effect of discoloration, minocycline was excluded (double antibiotic paste, DAP) or replaced with clindamycin, amoxicillin, or cefaclor. These drugs are mixed with saline or propylene glycol until they form a thick creamy mixture with certain physical consistency (approximately 1 mg/ml). At this concentration, however, TAP appears to have long-lasting deleterious effects on stem cell survival, so the dilution of DAP or TAP to 0.01‐0.1 mg/ml that appears as a liquid and is no longer a paste is recommended to retain the desirable anti-bacterial and anti-biofilm effect and avoiding stem cell toxicity. Furthermore, this undesirable effect can be avoided greatly by the use Ca(OH)2 as an intracanal medicament. Ca(OH)2 is widely available, and is antimicrobial at concentrations that do not induce stem cell toxicity. However, case reviews have shown that root development may not be as prominent in Ca(OH)2 cases as in TAP cases.
 Many other frontiers have been investigated to establish biocompatible disinfection strategies that provide the most effective disinfection and prepare the root canal environment to promote periapical healing and regrowth of host tissues in the canal by the stem/progenitor cells in periapical tissues. These involve using nanoparticles and tissue engineering strategies that include the evaluation of suitable scaffolds, growth factors, and harvested stem cells to be used in pulp-dentin regeneration. These developments in the regeneration of a functional pulp-dentin complex are a promising step towards the regeneration of destroyed dental tissues that ultimately retain the natural dentition, which is the ultimate goal of our endodontic practice.
Many other frontiers have been investigated to establish biocompatible disinfection strategies that provide the most effective disinfection and prepare the root canal environment to promote periapical healing and regrowth of host tissues in the canal by the stem/progenitor cells in periapical tissues. These involve using nanoparticles and tissue engineering strategies that include the evaluation of suitable scaffolds, growth factors, and harvested stem cells to be used in pulp-dentin regeneration. These developments in the regeneration of a functional pulp-dentin complex are a promising step towards the regeneration of destroyed dental tissues that ultimately retain the natural dentition, which is the ultimate goal of our endodontic practice.
References
- American Association of Endodontists. AAE clinical considerations for a re- generative procedure. 2016. Available at: https://www.aae.org/uploadedfiles/ publications_and_research/research/currentregenerativeendodonticconsiderations. pdf. Accessed June 8, 2016.
- Galler KM, Krastl G, Simon S, et al. European Society of Endodontology position statement: Revitalization procedures. Int Endod J 2016;49:717–23.
- Jeeruphan T, Jantarat J, Yanpiset K, et al. Mahidol study 1: comparison of radiographic and survival outcomes of immature teeth treated with either regenerative endodontic or apexification methods—a retrospective study. J Endod. 2012;38(10):1330-1336.
- Almutairi W, Yassen GH, Aminoshariae A, Williams KA, Mickel A. Regenerative Endodontics: A Systematic Analysis of the Failed Cases. J Endod. 2019;45(5):567-77.
- Galler KM, D’Souza RN, Federlin M, et al. Dentin conditioning codetermines cell fate in regenerative endodontics. J Endod. 2011;37(11): 1536-1541.
- Martin DE, De Almeida JF, Henry MA, et al. Concentration- dependent effect of sodium hypochlorite on stem cells of apical papilla survival and differentiation. J Endod. 2014;40(1):51-55.
- Galler KM, Buchalla W, Hiller KA, et al. Influence of root canal disinfectants on growth factor release from dentin. J Endod. 2015;41(3):363-368.
- Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod 2012;38:1372–5.
- Diogenes A, Hargreaves KM. Microbial Modulation of Stem Cells and Future Directions in Regenerative Endodontics. J Endod. 2017;43(9S):S95-S101
- Fouad AF. Microbial Factors and Antimicrobial Strategies in Dental Pulp Regeneration. J Endod. 2017;43(9S):S46-S50
Dr. Su-min Lee is assistant professor, Department of Endodontics, at University of Pennsylvania School of Dental Medicine. She can be reached at suminlee@upenn.edu.




