Targeted Endodontic Microsurgery
 Narrowing the Gap Between Novice and Adept
Narrowing the Gap Between Novice and Adept
Jarom Ray, D.D.S.
Thirteen thousand years ago, a hunter-gatherer received treatment described in the Journal of Physical Anthropology as “…consistent with tool‐assisted manipulation to remove necrotic or infected pulp in vivo and the subsequent use of a composite, organic filling (1).” Were this not to have occurred during the Paleolithic Era, the use of a file for root canal treatment would seem uninteresting, although the obturation concoction of bitumen, vegetable fibers and hair might raise an eyebrow. But this was a stone file! If alive today, the paleo-practitioner might be surprised to learn that customized 3D printed guides are being used in surgical endodontics, and that many obese hominids are returning to hunter-gatherer dietary principles (2,3). At any rate, in the summer of 2017 our team combined hollow cylindrical trephine burs traditionally used to remove implants, with a 3D-printed surgical guide for osteotomy and root-end resection of tooth #9 (Figure 1). The potential of the procedure became concrete when we successfully completed a second case, a flapless palatal approach to the palatal root of tooth #2 (Figure 2). We called the procedure Targeted Endodontic Microsurgery (TEMS).
The Essence of TEMS
The functional unit of TEMS is best described as a tube within a tube. The outer tube is attached to the body of a surgical guide in an orientation prescribed during virtual design. The inner tube is a hollow trephine with cutting teeth at its terminus. After flap reflection, the custom surgical guide is seated upon the patient’s dentition. The trephine is inserted and incrementally tapped through bone and root end at 1,000 rpm while being flooded by sterile water. Once the prescribed depth is reached, the trephine and guide are removed and a cylindrical core containing bone, root end and soft tissue lesion is elevated. Osteotomy depth, diameter, and angulation are customized to accommodate the patient’s anatomy before the first incision is made (Figure 3).
The Digital Workflow Overview
Our 3D printing lab routinely fabricates guides for implant placement, which made it relatively easy for us to adopt an existing digital workflow. We first digitize bone, roots and neurovascular spaces with CBCT. We use a 60 x 60 or 80 x 80 mm field of view depending upon the clinical scenario. In addition, it is necessary to digitize tooth surface anatomy that is obscured by CBCT scatter, and soft tissue contours that CBCT does not render. This is done with an impression that is sent to the lab to be poured in green stone and scanned with a desktop optical scanner. As an alternative to making an impression, we can perform a direct intraoral optical scan of tooth surfaces and intraoral soft tissues.
We send the two files to the lab, one from CBCT and one from an optical scan of the patient or cast. The files are uploaded to design software where they are merged to form a 3D construction containing a digital model. The trephine is measured with digital calipers and dimensions are input into implant design software. A prospective TEMS path with appropriate trephine dimensions appears on the screen. We spend a few minutes customizing the path for osteotomy and resection and approving the guide design. This step can occur virtually. Graduates of our program at distant locations are able to complete the digital workflow and their guides are mailed to them from our lab. TEMS adds about 10 minutes to our presurgical appointment—the time it takes to make an impression. TEMS requires 5 minutes of interaction with our lab for each root resection.
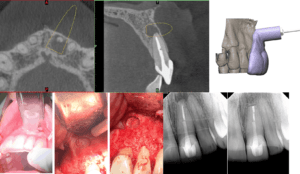
Figure 1: Tooth #9 with 10-month recall.
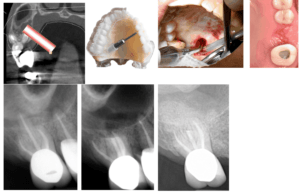
Figure 2: Tooth #2 with 18-month recall.
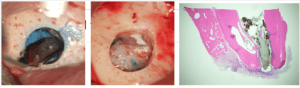
Figure 3: Tooth #30 osteotomy and resection. Photomicrograph of resected root end.
TEMS in Context With Other Technology
Over time, our specialty has become more capable and agile in part because of technological advances. We approach new technologies with skepticism, lest we be taken in by the trappings of pseudoscience. But if proven, we are moved to acquire the expertise and armamentarium necessary to bring additional tooth-saving capacity to our patients. Before clinicians adopted CBCT, they might have read several papers, talked with colleagues and attended presentations. Eventually clinicians made a large financial investment by purchasing a unit, complete with installation and on-sight instruction. Today CBCT is a key part of endodontic curriculum and practice.
As with CBCT, embracing digital endodontics will require an initial time investment. Major equipment purchases and extensive armamentarium additions are not necessary. In addition to learning how to use the trephine-guide combination clinically, endodontists will need to revive impression making capability, learn the basics of digital workflow and engage meaningfully with a 3D printing resource. Hopefully, endodontic-specific digital applications and armamentarium will be developed to further streamline the process.
Narrowing the Gap Between Novice and Adept
If a 2009 survey holds for today, 34% of endodontists are referring some endodontic surgery to an oral surgeon (4). Some endodontists might need an extra measure of confidence to engage surgical practice. For them, the security that comes with preplanning the osteotomy and resection, as well as the clinical time savings might be attractive. Guides narrow the gap between the adept and the novice. One of our team member’s first-ever apical surgery was a flapless palatal approach to the palatal root of tooth #14. Another’s second endodontic surgery was a flapless palatal approach to a maxillary 2nd molar. A novice surgeon capable of executing a digital workflow, can safely resect amenable root ends that traditionally were not attempted.
Although there is no evidence to suggest trephine resections affect root ends differently than traditional methods, well-designed ex vivo comparisons and clinical outcomes assessments are necessary to definitively answer this question. Our current recall x-rays and photomicrographs of root tips seem promising (Figure 3). Will TEMS become a sound link in the chain of endodontic advancement that began at least 13,000 years ago? That remains to be seen.
Dr. Ray has no conflict of interest related to the contents of this manuscript. Dr. Ray is an inventor listed on U.S. Application No. 16/396,185, filed April 26, 2018. All rights, title, and interest have been assigned to the Government of the United States. One or more embodiments of the inventions described in these patent applications are described in this manuscript. The views expressed are those of the author’s and do not reflect the official views or policy of the United States Department of Defense or the Uniformed Services University of the Health Sciences.
References:
(1) Oxilia G, Flavia F, et al. The Dawn of Dentistry in the late Upper Paleolithic: An early case of pathological intervention at Riparo Fredian. American Journal of Physical Anthropology 2017; 163:446-461.
(2) Giacomino C, Ray J, Wealleans J. Targeted Endodontic Microsurgery: A Novel Approach to Anatomically Challenging Scenarios Using 3-dimensional-printed Guides and Trephine Burs-A Report of 3 Cases. J Endod 2018;44:671-77.
(3) Anderson J, Wealleans J, Ray J. Endodontic Applications of 3D Printing. International Endodontic Journal 2018;51:1005-18.
(4) Creasy JE, Mines P, Sweet M. Surgical Trends Among Endodontists: The Result of a Web-based Survey. J of Endod 2009;35:30-34.
Dr. Jarom Ray is the residency program director at the Air Force Postgraduate Dental School and serves as associate professor of endodontics at the Uniformed Services University of the Health Sciences. You can catch his presentation, “3D Printing Guides for Targeted Endodontic Microsurgery (TEMS)”, on November 14, 2019, at Insight Track: New Technologies in Endodontics. Register at aae.org/insighttrack.




