COS-ENDO: Elevating Evidence-Based Endodontics Through Standardized Outcomes
First and Corresponding Author: Amir Azarpazhooh, DDS, MSc, PhD, FRCP(C).
Second Author: Maryam Zanjir, DDS, MSc.
Why Standardizing Endodontic Outcomes Matters
As clinicians, our goal is to provide the best care guided by high-quality evidence. Yet, challenges remain due to variations in study design, inconsistent outcome measures, and reporting bias that limit undertaking systematic reviews and meta-analyses. This weakens the translation of research into clinical practice. These issues are especially clear in endodontics, where treatments span all age groups but outcome reporting lacks consistency. Standardizing outcomes by defining the minimum to measure across studies will reduce variability, improve evidence synthesis, support clinical decision-making, and ultimately enhance patient-centered care (1, 2).
AAE’s Two-Phase Initiative: Leading with Evidence
Recognizing the need for standardized outcomes, the American Association of Endodontists (AAE) and its Foundation for Endodontics established a Special Committee to develop an Outcomes Consensus Conference. Following two requests for applications, our team at the University of Toronto submitted two proposals (in July 2019 and in April 2023) and was honored to receive both grants.
- Phase 1 involved a comprehensive scoping review of endodontic treatment outcomes from studies on Nonsurgical Root Canal Treatment, Retreatment, and Apexification over the past four decades. Published in three papers in the Journal of Endodontics (2-4), the review revealed significant inconsistencies limiting research comparability and interpretation.
- Phase 2 focused on developing the Core Outcome Set in Endodontics (COS-ENDO). Led by a Steering Committee of 6 clinicians (5 endodontists, 1 general dentist), 3 methodologists (including a COMET consultant), 2 patients with endodontic treatment experience, and 2 research coordinators, this diverse team ensured a thorough, patient-informed process. They oversaw all stages, from protocol development to survey design, study conduct, and outcome review. The results, published in five papers in the Journal of Endodontics (5-9), established the minimum set of core outcomes to be measured in studies of Nonsurgical Root Canal Treatment, Retreatment, Surgical Endodontics, Vital Pulp Therapy, Apexification, and Regenerative Endodontics.
How COS-ENDO Was Developed
COS-ENDO was developed using rigorous methods based on the COMET Handbook (1). The process included identifying outcomes from scoping reviews, qualitative interviews with patients (10), and a survey of practicing AAE endodontists. Key stakeholders—patients, clinicians, and researchers—participated in a two-round Delphi survey and then a consensus meeting to assess the importance of each outcome, considering its relevance to health or treatment, comparative importance, and whether it should be measured in every study.
- In the first round, 73 participants—24 academicians/researchers, 26 clinicians, and 23 patients/caregivers—rated outcomes on a scale from 1 (not important) to 9 (critically important) and could suggest new outcomes. Any new outcomes proposed by at least two participants were added to the second round. To ensure unbiased feedback, responses were anonymous and summarized by stakeholder group.
- In the second round, 70 participants reviewed the overall results, revised ratings if desired, explained changes in ratings, and indicated their availability for the final consensus meeting.
- A virtual consensus meeting was held on May 12, 2024, via Zoom to finalize COS-ENDO. Sixteen voting participants who completed both rounds received materials in advance and took part in one-on-one sessions to clarify objectives prior to the meeting. Moderated by an independent Chair, the meeting reviewed outcomes agreed for inclusion or exclusion, discussed those requiring further consideration, and rerated outcomes lacking consensus based on pre-defined criteria. Voting was anonymous, conducted through Zoom polls, with immediate result disclosure.
What Outcomes Made the Core List?
Through this consensus process, a core set of outcomes was prioritized for all endodontic treatments. These include tooth survival, pain, signs of infection, radiographic evidence of periradicular healing (or maintained health), success, functional tooth, need for further intervention, and adverse events or complications. For Vital Pulp Therapy, Apexification, and Regenerative Endodontics, continued root development was also included. These outcomes were classified using the framework by Dodd et al. (11) to promote consistent reporting and facilitate data retrieval (Figure 1). Dodd’s taxonomy categorizes outcomes based on the impact of interventions in five core areas: Mortality/Survival (survival rates); Physiological/Clinical (clinical signs and functions); Life Impact (effects on daily life and quality of life); Resource Use (economic factors and additional interventions or medications); and Adverse Events/Effects (complications and unintended consequences). This framework ensures comprehensive, standardized outcome reporting relevant to both clinicians and patients.
Patient-Centered by Design
Every outcome included in COS-ENDO was prioritized by patients and reflects what matters most to them, based on Fletcher’s 6Ds model: death, disease, discomfort, disability, dissatisfaction, and destitution (12). Discussing these outcomes with patients fosters open communication, improves diagnosis, and supports personalized care. By focusing on what patients value and measuring it consistently, clinical results improve, expectations are met, and trust is strengthened, leading to better outcomes and stronger therapeutic relationships.
Why COS-ENDO Matters in Practice
Adopting COS-ENDO is not just for researchers. Clinicians who consistently measure these outcomes contribute to building stronger evidence, shaping clinical guidelines, supporting evidence-based decisions, and enhancing patient-centered care. Integrating COS-ENDO into education, research training, and conferences will increase awareness and promote its adoption across the profession (1, 13). Clinicians can start by collecting core outcomes during treatment and follow-up visits. While some validated tools are available, further work is needed to standardize measurement instruments. Even routine outcome collection can generate valuable datasets that strengthen the specialty.
The Bottom Line
COS-ENDO provides a foundation for a more standardized, transparent, and patient-centered approach to endodontic care. Its implementation across research and clinical settings will elevate the specialty and ensure the delivery of high-quality care supported by robust evidence. This study lays the groundwork for advancing endodontic research, with the next step being the development of a Core Outcome Measurement Set. Following COMET guidelines (1, 14), this will involve reaching consensus on appropriate tools, timing, metrics, and aggregation methods; and ensuring the validity, reliability, responsiveness, and feasibility of measurement tools. Additionally, measuring contextual factors such as confounders and effect modifiers is crucial to explain variations in outcomes and enhance generalizability (15).
COS-ENDO classified following Dodd et al.’s taxonomy by core areas and domains. COS-ENDO, Core Outcome Set in Endodontics.
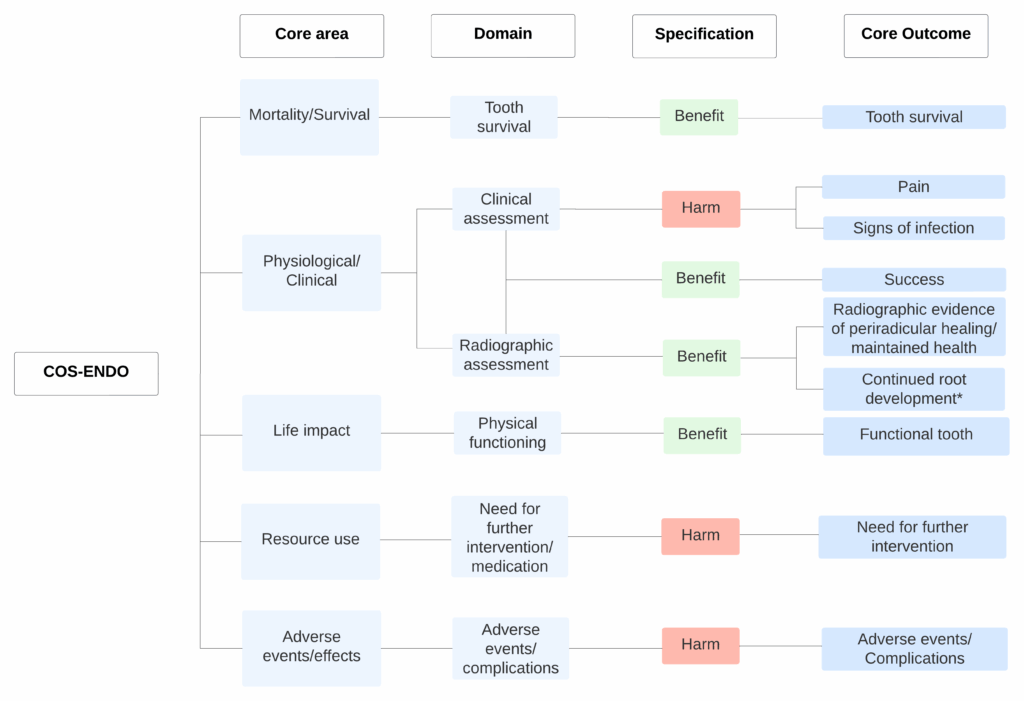
*Only applicable to Vital Pulp Therapy, Apexification and Regenerative Endodontics.
References
- Williamson PR, Altman DG, Bagley H, et al. The COMET Handbook: version 1.0. Trials. 2017;18(Suppl 3):280.
- Azarpazhooh A, Sgro A, Cardoso E, et al. A Scoping Review of 4 Decades of Outcomes in Nonsurgical Root Canal Treatment, Nonsurgical Retreatment, and Apexification Studies-Part 2: Outcome Measures. J Endod. 2022;48(1):29-39.
- Azarpazhooh A, Cardoso E, Sgro A, et al. A Scoping Review of 4 Decades of Outcomes in Nonsurgical Root Canal Treatment, Nonsurgical Retreatment, and Apexification Studies-Part 1: Process and General Results. J Endod. 2022;48(1):15-28.
- Azarpazhooh A, Khazaei S, Jafarzadeh H, et al. A Scoping Review of Four Decades of Outcomes in Nonsurgical Root Canal Treatment, Nonsurgical Retreatment, and Apexification Studies: Part 3-A Proposed Framework for Standardized Data Collection and Reporting of Endodontic Outcome Studies. J Endod. 2022;48(1):40-54.
- Azarpazhooh A, Zanjir M, Cardoso E, et al. Development of a Core Outcome Set in Endodontics (COS-ENDO): Part 1-General Methods for Developing COS-ENDO for Studies of Nonsurgical Root Canal Treatment, Retreatment, Surgical Endodontics, Vital Pulp Therapy, Apexification, and Regenerative Endodontics in Permanent Teeth. J Endod. 2025;51(4):401-11.
- Zanjir M, Cardoso E, Harman NL, et al. Development of a Core Outcome Set in Endodontics (COS-ENDO). Part 2: COS-ENDO for Studies of Nonsurgical Root Canal Treatment and Retreatment in Permanent Teeth. J Endod. 2025;51(4):412-26.
- Zanjir M, Cardoso E, Harman NL, et al. Development of a Core Outcome Set in Endodontics (COS-ENDO): Part 3 – COS-ENDO for Studies of Surgical Endodontics in Permanent Teeth. J Endod. 2025;51(4):427-41.
- Zanjir M, Cardoso E, Harman NL, et al. Development of a Core Outcome Set for Endodontics (COS-ENDO). Part 4: COS-ENDO for Studies of Vital Pulp Therapy in Permanent Teeth. J Endod. 2025;51(4):442-56.
- Zanjir M, Cardoso E, Harman NL, et al. Development of a Core Outcome Set for Endodontics (COS-ENDO). Part 5: COS-ENDO for Studies of Apexification and Regenerative Endodontics in Permanent Teeth. J Endod. 2025;51(4):457-72.
- Zanjir M, Azarpazhooh A, Hosseini Y, et al. Process-related Factors Are as Important as Outcomes for Patients Undergoing Nonsurgical Root Canal Treatment, Nonsurgical Root Canal Retreatment, and Endodontic Microsurgery. J Endod. 2023;49(10):1289-98.
- Dodd S, Clarke M, Becker L, et al. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol. 2018;96:84-92.
- Fletcher RH, Fletcher SW, Fletcher GS. Clinical epidemiology: the essentials: Lippincott Williams & Wilkins; 2014. p 2-4.
- Leshem YA, Simpson EL, Apfelbacher C, et al. The Harmonising Outcome Measures for Eczema (HOME) implementation roadmap. Br J Dermatol. 2023;189(6):710-8.
- Prinsen CA, Vohra S, Rose MR, Boers M, Tugwell P, Clarke M, et al. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” – a practical guideline. Trials. 2016;17(1):449.
- Boers M, Kirwan JR, Wells G, et al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol. 2014;67(7):745-53.
Dr. Amir Azarpazhooh can be reached at Amir.azarpazhooh@dentistry.utoronto.ca.
Disclaimer
The views and opinions expressed by authors are solely those of the authors and do not necessarily reflect the official policy or position of the American Association of Endodontists (AAE). Publication of these views does not imply endorsement by the AAE.
