Effects of Diabetes on Elemental Levels and Nanostructure of Root Canal Dentin
By M. A. Saghiri, DEng, MS, PhD
Abstract:
Diabetes mellitus induces significant changes in root canal dentin, altering both its mineral content and nanoscale crystal organization. These modifications influence the precision of canal shaping, the effectiveness of adhesive bonding, and the long-term prognosis of endodontic treatments. Despite growing evidence of these substrate differences, most clinical protocols remain uniform for all patients. It reviews the key chemical and structural alterations observed in diabetic dentin and presents practical recommendations for integrating a systemic-aware workflow to improve safety, efficacy, and treatment outcomes.
Introduction:
Successful endodontic therapy depends on predictable dentin properties to guide instrumentation, ensure reliable adhesion, and achieve a fluid-tight seal. In patients with diabetes mellitus, chronic hyperglycemia disrupts mineral homeostasis and leads to non-enzymatic glycation and oxidative stress that affect dentin microstructure1. As the global prevalence of diabetes continues to rise, clinicians must recognize and adapt techniques to address the unique characteristics of diabetic dentin2. A proactive, systemically informed approach that accounts for medical conditions can help reduce treatment risks.
Most clinical protocols overlook structural and chemical changes in dentin caused by diabetes. Reduced trace minerals, altered collagen and disrupted crystal organization can weaken dentin, impair smear layer formation and reduce bonding effectiveness3-5. These changes may increase the risk of procedural errors and restorative failure if not addressed. They can also affect how dentin responds to instrumentation, irrigation and obturation materials6. As a result, untreated variations in diabetic dentin may compromise both immediate and long-term treatment success. The purpose of this study is to examine these alterations in diabetic root dentin and to propose practical adjustments that support safer and more effective endodontic treatment in patients with diabetes.
Methods:
This study was conducted in accordance with the ethical guidelines approved by the Institutional Review Board at Rutgers School of Dental Medicine (Protocol No. Pro2019002923). A total of 20 extracted human molars were collected, anonymized, disinfected in 0.1% thymol, and stored at 4°C. All teeth were used within three months of extraction. The samples were evenly divided into two groups: individuals with type 2 diabetes mellitus (n = 10) and non-diabetic controls (n = 10). The diabetes group included individuals with a diagnosis of at least five years and HbA1c values of 7.0% or lower, recorded within three months of extraction7. Age- and sex-matching was applied across groups. Information about diabetes medications such as insulin or metformin was recorded when available but was not used as a selection criterion. Each tooth was sectioned at the cementoenamel junction, and dentin discs were prepared from the mid-root region (5–7 mm from the apex) using a low-speed diamond saw under continuous water irrigation7. The discs were rinsed with deionized water and stored in sealed, contamination-free tubes until analysis.
Elemental analysis was performed using inductively coupled plasma mass spectrometry (ICP-MS; Agilent 7900, USA) following acid digestion in high-purity nitric acid (70% HNO₃, Fisher Optima). Each sample was analyzed in triplicate. Calibration was performed using certified multi-element standards (Inorganic Ventures), and elements measured included lithium, magnesium, manganese, zinc, strontium, copper, and selenium8, 9. Detection limits were 0.5 parts per billion (ppb) for lithium and 1.2 ppb for selenium. Statistical analysis was conducted using SPSS version 27.0 (IBM Corp.). Group comparisons were performed using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test. Statistical significance was defined as p < 0.05. Confidence intervals (95%) and Cohen’s d effect sizes were also calculated.
Findings:
Our comparative analysis of molar dentin from well-controlled diabetic donors and matched non-diabetic controls revealed two primary alterations. First, diabetic dentin showed a 20 to 35% reduction in key trace minerals, magnesium, zinc, strontium, lithium, manganese, and selenium, with a 15% increase in copper levels. Since magnesium and zinc support crystal hardness and strontium and selenium contribute to antimicrobial defense, their depletion paired with higher copper (a marker of oxidative stress) creates a chemically distinct substrate with compromised mechanical and biological properties10, 11 (Fig 1) illustrates the loss of Mg²⁺/Zn²⁺ and concurrent enrichment of Cu²⁺ in the diabetic dentin lattice compared to healthy dentin. Second, imaging analysis showed diabetic dentin contains 2.5 times more mineral crystallites per unit area. However, each crystallite is 20 to 30% smaller and arranged in a less compact, mixed polycrystalline and amorphous network. This finer, heterogeneous scaffold increases surface area while weakening intercrystalline bonds, making the dentin more vulnerable to procedural damage and less receptive to adhesive infiltration12.
Clinical Implications:
The softer, heterogeneous nature of diabetic dentin raises the risk of procedural errors. Rotary or reciprocating files may overcut or deviate in areas of reduced hardness, leading to ledges, canal transportation, or perforations13. Altered smear layer characteristics and ion deficiencies can undermine resin infiltration and polymerization, increasing the potential for microleakage and restoration failure. The weakened crystal network increases susceptibility to vertical root fractures (VRFs), as reduced crystallite size and disordered mineral alignment have been linked to diminished fracture resistance in dentin14. A conservative recall schedule of every 6 to 12 months is advised to monitor restoration integrity and early signs of failure, although long-term clinical data in diabetic populations remain limited.
Recommended Protocol Adjustments:
Incorporating systemic health considerations into endodontic treatment protocols is essential for optimizing outcomes in patients with diabetes mellitus (Fig 2) shows each dentin change next to the matching treatment step for easier comparison. Risk stratification should begin with documentation of the patient’s diabetes status, including HbA1c levels and disease duration, followed by a clear discussion of how these systemic factors may compromise dentin structure and influence clinical decision-making. Access preparation must be deliberately conservative, providing sufficient visibility and straight-line canal access while minimizing unnecessary removal of pericervical dentin. A manual glide path should be established using size 10 or 15 stainless-steel hand files prior to introducing rotary instruments. During shaping, rotary files should be operated at 150–200 rpm with a torque limit of 1.0–1.5 N·cm to reduce stress on dentin that may already be weakened by microstructural alterations associated with chronic hyperglycemia6. Irrigation protocols should include the use of 17% EDTA for no longer than 1–2 minutes to avoid excessive demineralization, followed by a thorough saline rinse15. To minimize the risk of microcrack propagation in brittle dentin, irrigant activation should employ low-frequency sonic devices (1–2 kHz) rather than high-powered ultrasonics16. During obturation, the use of bioactive, ion-releasing sealers that liberate magnesium, strontium, or zinc may support remineralization and enhance dentin stability17, 18. Adhesive strategies should be adapted to diabetic substrates by selectively removing the smear layer, utilizing primers containing functional monomers such as 10-MDP, and maintaining a moist dentin surface to facilitate optimal resin infiltration19. Final restorative planning must emphasize ferrule preservation and cuspal coverage to distribute occlusal forces and prevent structural failure. Radiographic follow-up at 6- to 12-month intervals is recommended to monitor healing and detect early signs of post-treatment complications, particularly in patients with suboptimal glycemic control20.
Future Directions:
Future research should focus on developing tailored biomaterials such as sealers with controlled ion-release kinetics or nanoparticle additives to reinforce crystal networks. Instrument manufacturers could design novel alloys and file geometries specifically for low-hardness substrates. Well-designed clinical trials comparing systemic-aware workflows to conventional protocols will be essential to validate these approaches and measure technical outcomes as well as patient-centered metrics like postoperative sensitivity and tooth survival.
Conclusion:
Diabetes causes profound chemical and structural changes in root canal dentin. Recognizing these alterations and adopting a systemic-aware endodontic protocol, including risk assessment, conservative access, gentle shaping, controlled irrigation, bioactive materials, enhanced bonding, and protective restorations, can reduce procedural complications, improve adhesive performance, and extend the functional lifespan of endodontically treated teeth in diabetic patients.
Figures:
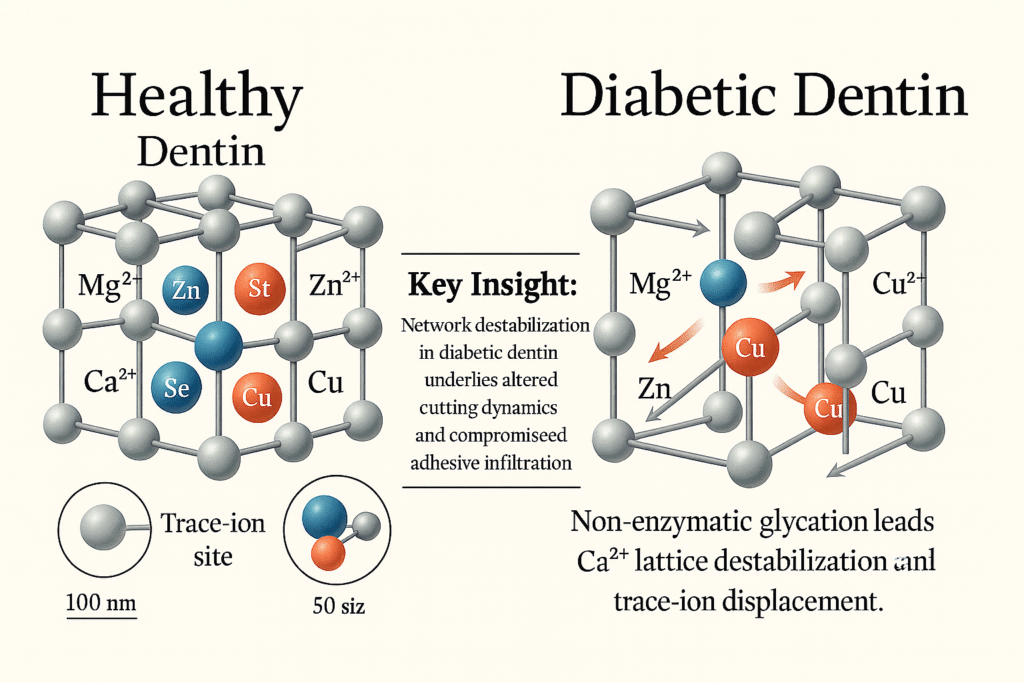 Figure 1. Ion Displacement and Lattice Destabilization in Diabetic Dentin
Figure 1. Ion Displacement and Lattice Destabilization in Diabetic Dentin
This image compares healthy and diabetic dentin at the nanoscale. Healthy dentin has a stable calcium network with trace ions such as magnesium, zinc, strontium, selenium, and copper, which are evenly embedded and support strong cutting and reliable bonding. In diabetic dentin, high blood sugar causes nonenzymatic glycation that breaks calcium links, forcing out magnesium and zinc and allowing extra copper to fill the gaps. This disruption makes the dentin more brittle, produces uneven debris during cutting, and weakens sealer bonding.
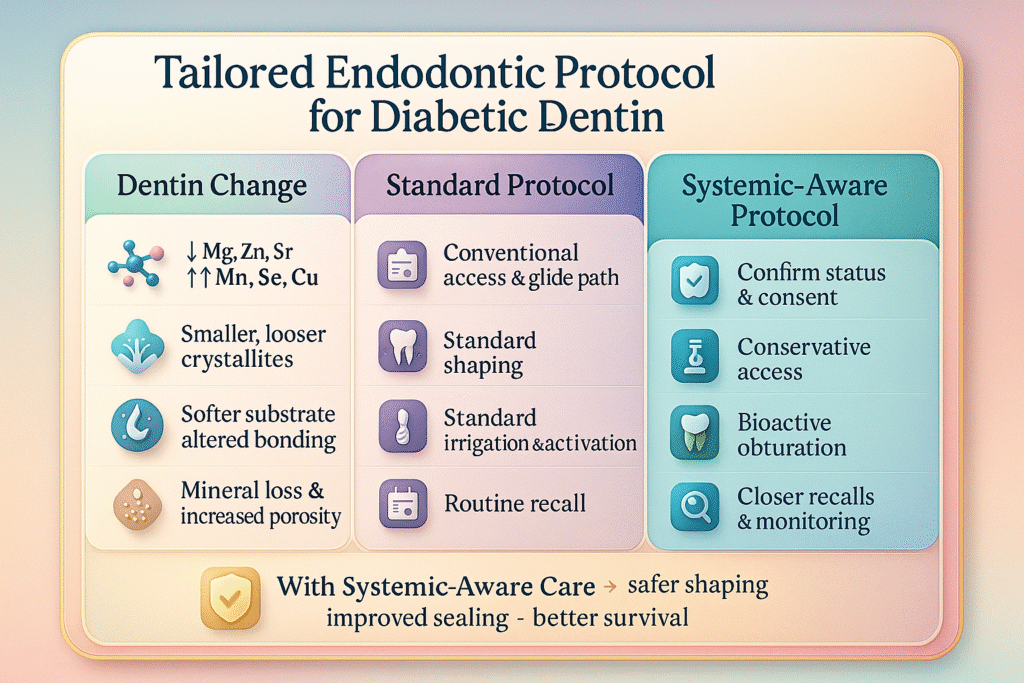 Fig. 2. Systemic-Aware Endodontic Protocol for Diabetic Dentin
Fig. 2. Systemic-Aware Endodontic Protocol for Diabetic Dentin
From left to right, the figure assesses the patient’s medical history and obtains informed consent, prepares a conservative access cavity to protect compromised dentin, employs gentle low-torque instrumentation for canal shaping, places a bioactive sealer with activated irrigation to maximize disinfection and seal integrity, and arranges more frequent follow-up visits for early detection of leaks or fractures.
References:
- Bueno EM, Glowacki J. Biologic foundations for skeletal tissue engineering: Morgan & Claypool Publishers; 2011.
- Kidambi S, Patel SB. Diabetes mellitus: considerations for dentistry. The Journal of the American Dental Association. 2008;139:8S-18S.
- Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: Structure, Composition and Mineralization: The role of dentin ECM in dentin formation and mineralization. Frontiers in bioscience (Elite edition). 2011;3:711.
- Vital SO, Gaucher C, Bardet C, Rowe P, George A, Linglart A, et al. Tooth dentin defects reflect genetic disorders affecting bone mineralization. Bone. 2012;50(4):989-97.
- Saghiri MA, Vakhnovetsky J, Samadi E, Napoli S, Samadi F, Conte M, et al. Effects of Diabetes on Elemental Levels and Nanostructure of Root Canal Dentin. J Endod. 2023;49(9):1169-75.
- Saghiri MA, Aminsobhani M, Gutmann JL, Kawai T, Nath D, Hirschberg C. Effect of Diabetes on Rotary Instrumentation of Dentin. J Endod. 2021;47(8):1301-7.
- Saghiri MA, Rahmani B, Conte M, Nath D, Peters O, Morgano S. Diabetes Mellitus Affects the Microhardness of Root Dentine: An in-vitro Study. Eur Endod J. 2022;7(2):122-8.
- Attinger D, Moore C, Donaldson A, Jafari A, Stone HA. Fluid dynamics topics in bloodstain pattern analysis: Comparative review and research opportunities. Forensic science international. 2013;231(1-3):375-96.
- Kumagai A, Fujita Y, Endo S, Itai K. Concentrations of trace element in human dentin by sex and age. Forensic science international. 2012;219(1-3):29-32.
- Shaik I, Dasari B, Shaik A, Doos M, Kolli H, Rana D, et al. Functional Role of Inorganic Trace Elements on Enamel and Dentin Formation: A Review. J Pharm Bioallied Sci. 2021;13(Suppl 2):S952-S6.
- Saghiri MA, Vakhnovetsky J, Vakhnovetsky A, Ghobrial M, Nath D, Morgano SM. Functional role of inorganic trace elements in dentin apatite tissue-Part 1: Mg, Sr, Zn, and Fe. J Trace Elem Med Biol. 2022;71:126932.
- Noohi P, Abdekhodaie MJ, Nekoofar MH, Galler KM, Dummer PM. Advances in scaffolds used for pulp–dentine complex tissue engineering: A narrative review. International endodontic journal. 2022;55(12):1277-316.
- Peters OA, Arias A. Rotary and reciprocating motions during canal preparation. Endodontic Advances and Evidence‐Based Clinical Guidelines. 2022:283-310.
- Patel S, Bhuva B, Bose R. Present status and future directions: vertical root fractures in root filled teeth. International Endodontic Journal. 2022;55:804-26.
- Wagner MH, Da Rosa RA, de Figueiredo JAP, Duarte MAH, Pereira JR, Só MVR. Final irrigation protocols may affect intraradicular dentin ultrastructure. Clinical oral investigations. 2017;21(7):2173-82.
- Sachdeva N, Nikhil V, Jha P. Effect of ultrasonic root-end cavity preparation on dentinal microcrack formation: A micro-computed tomography study. Journal of Conservative Dentistry and Endodontics. 2019;22(4):362-6.
- Pires PM, Neves AdA, Makeeva IM, Schwendicke F, Faus-Matoses V, Yoshihara K, et al. Contemporary restorative ion-releasing materials: Current status, interfacial properties and operative approaches. British Dental Journal. 2020;229(7):450-8.
- AlGhannam MI, AlAbbas MaS, AlJishi JA, AlRuwaili MA, AlHumaid J, Ibrahim MS. Remineralizing effects of resin-based dental sealants: a systematic review of in vitro studies. Polymers. 2022;14(4):779.
- Pegado REF, do Amaral FLB, Flório FM, Basting RT. Effect of different bonding strategies on adhesion to deep and superficial permanent dentin. European journal of dentistry. 2010;4(02):110-7.
- Caussin E, Izart M, Ceinos R, Attal J-P, Beres F, François P. Advanced material strategy for restoring damaged endodontically treated teeth: a comprehensive review. Materials. 2024;17(15):3736.
