Endodontic Periodontic Lesions
By Ronald Ordinola-Zapata1, DDS, MS, PhD; JT Crepps1,2, DDS, MS; Blake Clarke1 , DDS
The relationship between the dental pulp and periodontium is complex and dynamic, exemplified by the occurrence of endodontic-periodontal lesions. These lesions can develop through various anatomical and iatrogenic pathways, including furcation canals, dentinal cracks, vertical root fractures, and cemental tears (1, 2).
Microbial biofilms, a multicellular population encased in an extracellular matrix (3), play a crucial role in all endodontic infections. These biofilms characteristically adhere to organic and inorganic substrates such as enamel, dentin or cementum (3-5). The root canal microbiome primarily consists of a mixed infection of anaerobic bacteria, many with highly proteolytic capabilities (6-10). See Figure 1.
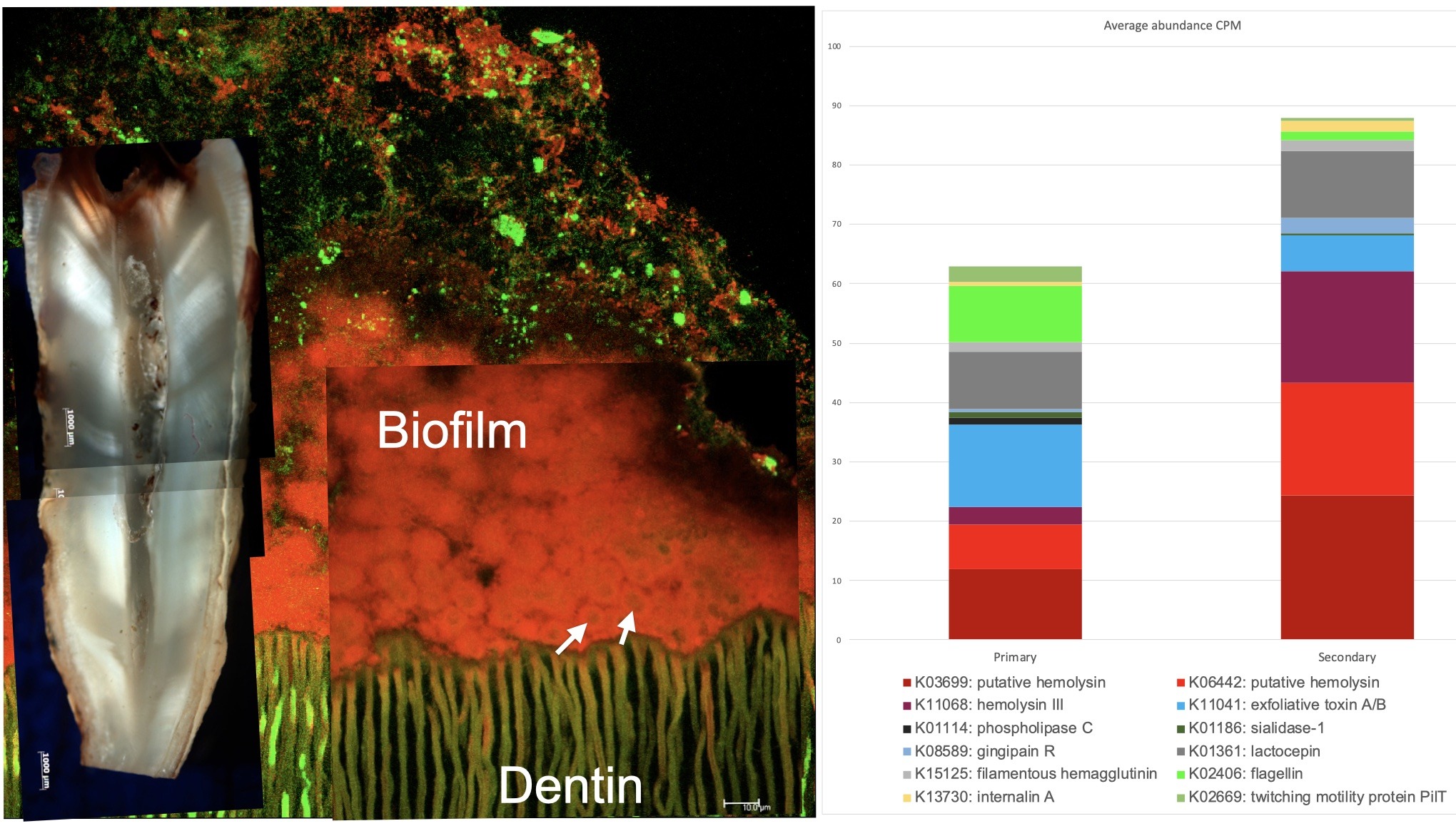
Figure 1. (Left) Confocal laser scanning microscopy microphotograph showing a typical biofilm architecture found in a case with pulp necrosis. The microbial cells are firmly attached to the dentin structure. Reproduced with permission from (Ordinola-Zapata et al. International Endodontic Journal 2022; 55: 613-616). Abundance of microbe associated molecular patterns found in primary and secondary endodontic infections. Data obtained from (9). (Right).
Biofilm-associated infections are caused by a complex interplay of microbial and enzymatic activity (11). Hemolysins, the most common microbial toxin in root canals (9), break down inflammatory tissue, allowing anaerobic bacteria to utilize reduced ferrous iron essential for microbial activity (9). Oral bacteria can produce harmful substances that trigger immune responses and promote further bacterial proliferation, establishing a cycle of infection and inflammation (12, 13). Importantly, bacterial byproducts can infiltrate various anatomical routes, facilitating the spread of infection and bone resorption between endodontic and periodontal tissues. This interconnection is key to understanding the development and progression of endodontic-periodontal lesions.
Communication pathways between a contaminated pulp canal space and the periodontium can be either physiological or iatrogenic. While the apical foramen is the most common pathway, accessory canals, furcation canals, and exposed dentinal tubules also provide routes for microbial toxins to diffuse into the periapical tissues and furcation area. These pathways are considered physiological (14). Another source of communication is caused by root damage. See Figure 2. Dentinal cracks, perforations, and vertical root fractures are usually contaminated which can also produce bone resorption (15-17). Furthermore, anatomical anomalies (i.e., palatogingival groove) can also influence the route bacterial byproducts take to reach the attachment apparatus or the pulp canal space.
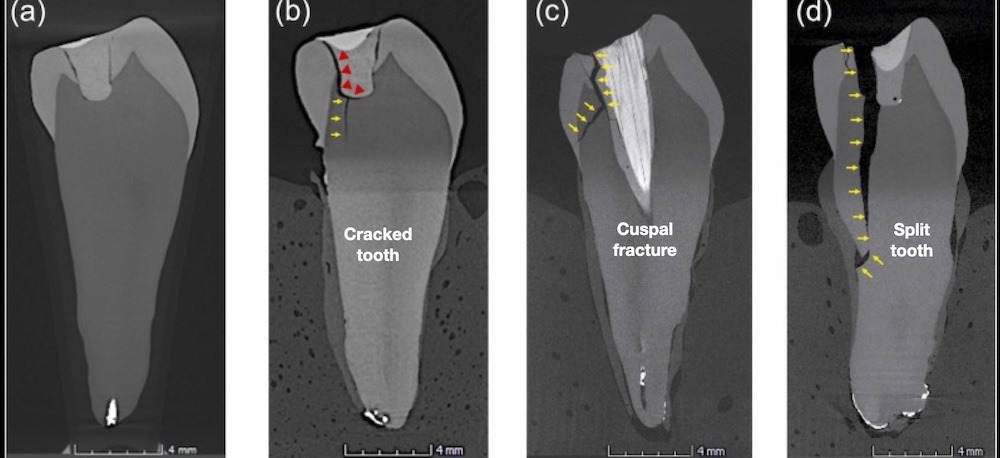
Figure 2. Microcomputed tomography sections showing debonding of the dentin-composite interface (a). A longitudinal crack in a mandibular premolar after cyclic loading (a-b) can propagate affecting one cusp (c); or result in a complex corono-radicular fracture (d). Reproduced with permission from (26).
Simon, Glick, and Frank (2), classified endodontic-periodontic lesions into primary endodontic lesions, primary endodontic lesions with secondary periodontal involvement, primary periodontal lesions, primary periodontal lesions with secondary endodontic involvement, and “true” combined lesions (18). Primary endodontic infections are characterized by pulp necrosis or untreated endodontic disease, which can lead to drainage adjacent to the gingival sulcus. This could create a tissue-destructive process which proceeds from the apical region towards the gingival margin (19). Radiolucencies in the furcation area of non-vital posterior teeth may indicate the presence of furcation canals or a crack located on the floor of the pulp chamber. Resorption at the furcation can also be the result of apical inflammation. Walton (15) found in animal experiments that the primary “movement” of apical lesions was towards the furcation in 53% of the cases. Jansson & Ehnevid (20) revealed that teeth that exhibited periapical pathology at both roots had significantly greater periodontal probing depths than teeth without periapical pathology. Also, furcation depths of higher than 3 mm were significantly more frequent in molars with periapical pathology (20). See Figures 3 and 4.
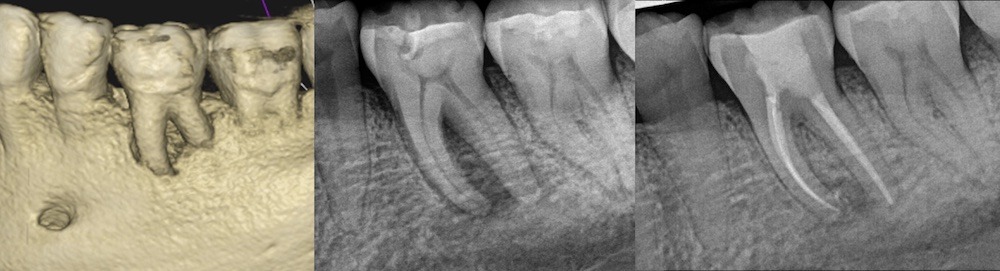
Figure 3. Patient presented with furcation involvement and loss of buccal plate extending apically on tooth #19 as shown on CBCT rendering (left) and pre-operative periapical radiograph (center). Treatment was initiated and Ca(OH)2 medication was placed – the patient return for obturation 4 months later, at which point marked healing of furcal bone was evident radiographically (right).
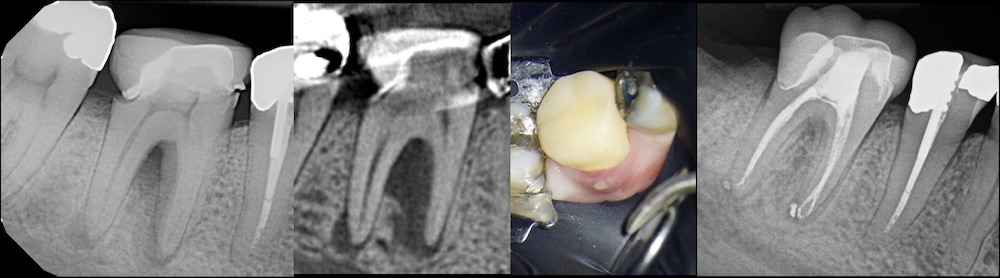
Figure 4. A primary endodontic lesion in a mandibular first molar. There is furcation involvement associated with a chronic apical abscess. Significant healing was observed at the recall appointment after non-surgical root canal treatment.
It is important to note that primary endodontic lesions occur exclusively in the presence of pulpal disease (2). Diagnostic measures such as sensibility testing, identification of mechanical allodynia, transillumination, and periodontal probing are critical for differentiating between primary endodontic lesions and primary periodontal lesions. Untreated primary endodontic lesions may also become secondarily involved with periodontal breakdown if plaque or calculus form at the gingival margin resulting in marginal periodontitis (2). Overall, healing of the endodontically induced areas could be anticipated but the prognosis also depends on periodontal treatment to remove the additional sources of inflammation.
While the impact of pulpal disease on the periodontium is well-established, the reverse relationship – the effect of periodontal disease on the pulp – has been the subject of considerable debate by numerous researchers. In the 1970s, Bergenholtz & Lindhe (21) performed a study on monkeys in which periodontal disease was induced in 92 permanent teeth. After six months, 30-40% of horizontal bone loss was observed. Histological analysis showed that 57% of the dental pulps presented secondary dentin formation, calcifications, and mild localized inflammation. However, only one case developed pulp necrosis (21). Overall, the chances for pulpal damage when periodontal disease is present are low, unless the periodontal pocket and subgingival plaque reaches the apical third and cementum damage is present (22).
Teeth with root damage can also produce endodontic periodontic lesions. A cracked tooth is defined as a thin surface disruption of enamel and dentin, and possible cementum of unknown depth of extension (23). In these cases, the coronal crack can establish a communication pathway between the oral environment, the pulp chamber and the periodontal ligament. See Figure 5. Bacterial colonization along the crack line is linked to pulpal inflammation and necrosis (24). Posterior teeth are subject to the same principles of materials science. In particular, they are subject to cyclic loading or fatigue failure, which is the most common form of mechanical failure (25-27). It is important to note that the force or stress required to initiate and propagate a crack decrease as the number of loading cycles increases. According to Arola & Reprogel (28) the flexural strength of coronal dentin decreases almost 20 MPa per decade of life due to the repetitive nature of masticatory forces, chances for crack formation and propagation increases progressively with aging. Eventually, the contaminated crack will extend to the pulp chamber (24) and, ultimately could face the crestal bone causing bone resorption changes.
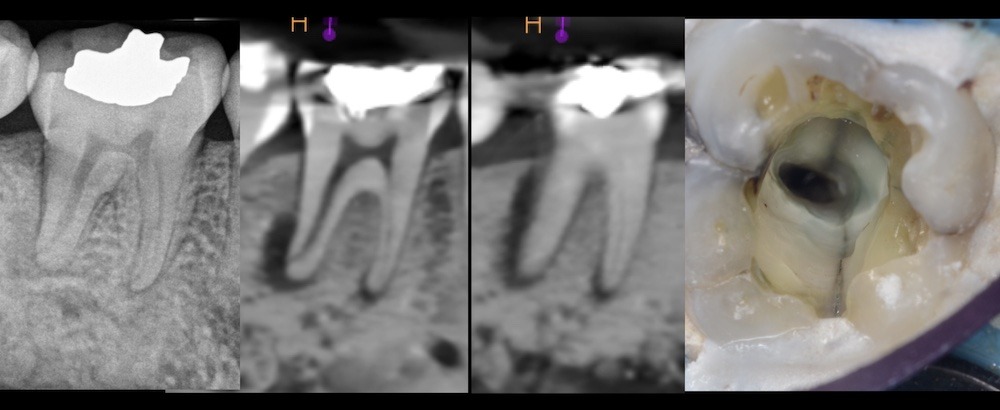
Figure 5. Pre-operative periapical (left) and CBCT slices (center) of tooth #19 showing bone resorption extending up the distal root. Upon access, a crack was visible extending into the distal canal orifice (right). Cracks are always colonized with bacterial biofilms (24).
Vertical root fractures are defined as a fracture in the root whereby the fractured segments are incompletely separated. See Figure 6. It may occur buccal-lingually or mesial-distally and often leads to an isolated periodontal defect or sinus tract (23). Potential irritants previously identified in these fractures are bacteria, necrotic tissue, and food debris (29). Mandibular molars (34%) and maxillary premolars (22%) are most frequently affected (30). Vertical root fractures appear to be more correlated with previously treated teeth than teeth with necrotic pulps (31). The communication pathway created by these fractures can lead to periodontal destruction adjacent to the root fracture. See Figure 7. Practitioners should be aware of the overlap in clinical presentation of vertical root fractures and other causes of endodontic-periodontal lesions. In previously treated teeth, clinicians typically suspect vertical root fractures, but they must consider the possibility of a chronic apical abscesses presenting as narrow, deep probing depths as a pathway of drainage through the sulcus. Although vertical root fractures may not be completely evident on CBCT scans, three-dimensional imaging allows the clinician to obtain other information that may be related to root canal treatment failure like missed canals, resorptive defects, strip perforations among others. Accurate diagnosis is crucial and prevents unnecessary treatment to the patient.
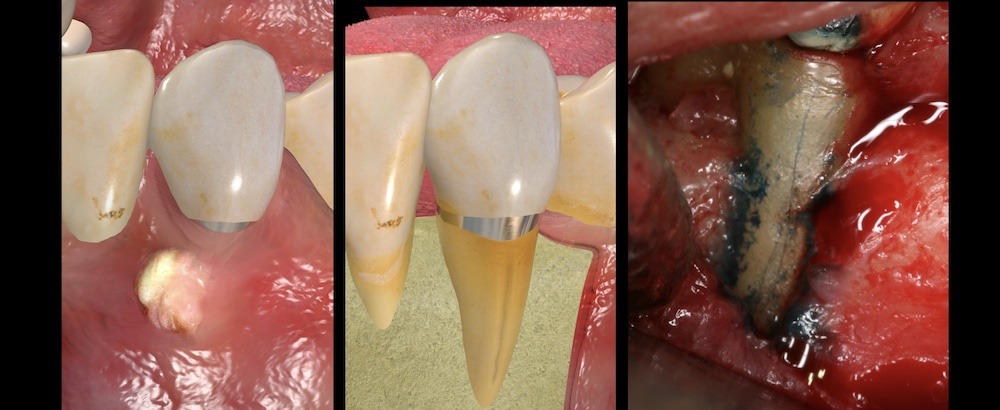
Figure 6. (Left) Three-dimensional rendering showing abscess on gingival tissues associated with a mandibular canine. Rendering showing a vertical root fracture and associated bone loss pattern (middle). Clinical view of a vertical root fracture after surgical exposure (Right).
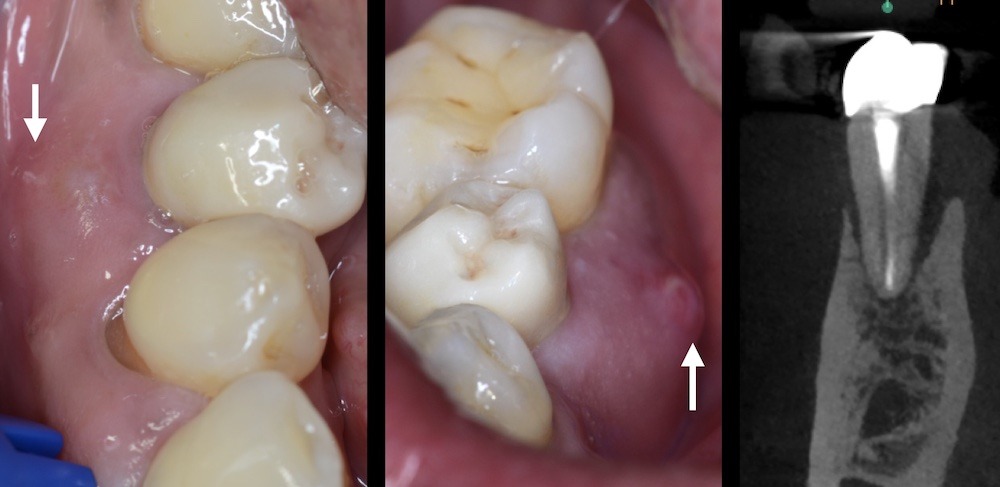
Figure 7. Tooth #29 presented with both buccal (left) and lingual (right) sinus tracts suggestive of a vertical root fracture. Observe the resorption of the buccal and lingual cortical plate.
Another factor associated with periodontal bone loss are cemental tears (32). This is a surface root fracture involving the cementum and sometimes the root dentin that occurs at the cemental dentinal interface (33). If the cemental tear is located at the apical level it could resemble an endodontic failure, at the middle level it will create a periodontal pocket or mimic periodontal disease (32-37). Cemental tears are more prevalent in incisors (74%) and are located on the interproximal surface (79%) (35). Seventy-one percent of patients with cemental tears are over 60 years old (34, 35). Overall, the prognosis of these cases is questionable. The surgical approach is more successful compared to the non-surgical one (57 vs 28%). Cases located at the cervical and middle third have better prognosis compared to cemental tears located at the apical third (60 vs 11%), (34). See Figure 8.
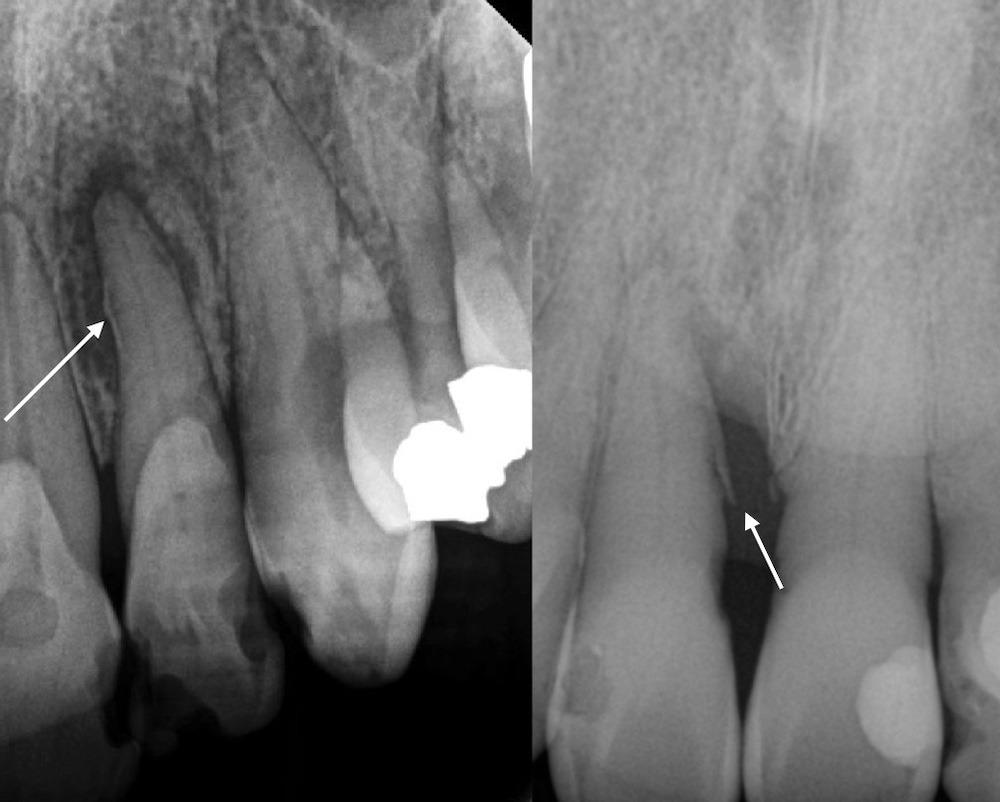
Figure 8. Cemental tears shown in periapical radiographs on the mesial of tooth #10 (left) and mesial of tooth #8 (right). Observe the associated bone loss pattern.
Treatment Approaches
Treatment approaches for endodontic-periodontal lesions vary based upon etiology. Primary endodontic lesions arise exclusively from an endodontic source and can be treated with endodontic treatment alone (1). Primary periodontal lesions with secondary endodontic involvement or true combined lesions require endodontic treatment first followed by appropriate periodontal treatment 1-2 months later after a periodontal re-evaluation (38). However, Gupta et al. (39) found in a randomized clinical trial that nonsurgical periodontal treatment may be performed simultaneously with endodontic treatment in the management of concurrent endodontic-periodontal lesions without communication. Therefore, the observation period after endodontic treatment may not be required. Treatment options for cracked teeth include placing glass-ionomer intraorifice barrier apical to the crack line (40), while those with vertical root fractures may undergo root amputation or extraction. Those with complex anatomical variations, such as a palatogingival grooves, may be best treated with intentional replantation or other surgical approaches (41).
Endodontic-periodontal lesions, regardless of cause, present a complex clinical scenario that can challenge even experienced practitioners. The dynamic between pulpal and periodontal tissues is influenced by microbial biofilms, anatomical variations and iatrogenic factors. Recognition of these various etiologies allows clinicians to develop targeted and evidence-based treatment plans that lead to better outcomes for patients, including multidisciplinary care with periodontists when indicated.
References
- Abbott PV, Salgado JC. Strategies for the endodontic management of concurrent endodontic and periodontal diseases. Aust Dent J 2009;54 Suppl 1:S70-85.
- Simon JH, Glick DH, Frank AL. The relationship of endodontic-periodontic lesions. J Periodontol 1972;43(4):202-208.
- Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet 2001;358(9276):135-138.
- Chavez de Paz LE. Redefining the persistent infection in root canals: possible role of biofilm communities. J Endod 2007;33(6):652-662.
- Ricucci D, Siqueira JF. Biofilms and Apical Periodontitis: Study of Prevalence and Association with Clinical and Histopathologic Findings. Journal of Endodontics 2010;36(8):1277-1288.
- Fabricius L, Dahlén G, Holm SE, Möller AJ. Influence of combinations of oral bacteria on periapical tissues of monkeys. Scand J Dent Res 1982;90(3):200-206.
- Sundqvist GK, Eckerbom MI, Larsson AP, Sjögren UT. Capacity of anaerobic bacteria from necrotic dental pulps to induce purulent infections. Infect Immun 1979;25(2):685-693.
- Siqueira JF, Rôças IN. Microbiology and treatment of acute apical abscesses. Clin Microbiol Rev 2013;26(2):255-273.
- Ordinola-Zapata R, Costalonga M, Dietz M, Lima BP, Staley C. The root canal microbiome diversity and function. A whole-metagenome shotgun analysis. Int Endod J 2024;57(7):872-884.
- Schuweiler D, Ordinola-Zapata R, Dietz M, Lima BP, Noblett WC, Staley C. Microbial diversity in primary endodontic infections: demographics and radiographic characteristics. Clin Oral Investig 2024;28(11):591.
- Costalonga M, Herzberg MC. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett 2014;162(2 Pt A):22-38.
- Hajishengallis G, Lamont RJ, Koo H. Oral polymicrobial communities: Assembly, function, and impact on diseases. Cell Host Microbe 2023;31(4):528-538.
- Hajishengallis G. Illuminating the oral microbiome and its host interactions: animal models of disease. FEMS Microbiol Rev 2023;47(3).
- Evans M. The endodontic-periodontal juncture: Where two worlds meet. An overview of endo-perio lesions. Aust Dent J 2023;68 Suppl 1:S56-S65.
- Walton RE, Garnick JJ. The histology of periapical inflammatory lesions in permanent molars in monkeys. J Endod 1986;12(2):49-53.
- Herrera D, Retamal-Valdes B, Alonso B, Feres M. Acute periodontal lesions (periodontal abscesses and necrotizing periodontal diseases) and endo-periodontal lesions. J Periodontol 2018;89 Suppl 1:S85-S102.
- Kuoch P, Bonte E. Endoperiodontal Lesions and Chicago’s New Classification of Periodontal and Peri-implant Diseases and Conditions. J Contemp Dent Pract 2020;21(7):798-802.
- Belk CE, Gutmann JL. Perspectives, controversies and directives on pulpal-periodontal relationships. J Can Dent Assoc 1990;56(11):1013-1017.
- Simring M GM. The Pulpal Pocket Approach: Retrograde Periodontitis. Journal of Periodontology 1964;35(1):22-48.
- Jansson LE, Ehnevid H. The influence of endodontic infection on periodontal status in mandibular molars. J Periodontol 1998;69(12):1392-1396.
- Bergenholtz G, Lindhe J. Effect of experimentally induced marginal periodontitis and periodontal scaling on the dental pulp. J Clin Periodontol 1978;5(1):59-73.
- Ricucci D, Siqueira JF, Rôças IN. Pulp Response to Periodontal Disease: Novel Observations Help Clarify the Processes of Tissue Breakdown and Infection. J Endod 2021;47(5):740-754.
- American Association of Endodontists AAE. Glossary of Endodontic Terms. Chicago; 2020.
- Ricucci D, Siqueira JF, Loghin S, Berman LH. The cracked tooth: histopathologic and histobacteriologic aspects. J Endod 2015;41(3):343-352.
- Ordinola-Zapata R, Fok ASL. Research that matters: debunking the myth of the “fracture resistance” of root filled teeth. Int Endod J 2021;54(3):297-300.
- Lin F, Ordinola-Zapata R, Ye N, Xu H, Fok ASL. Fatigue analysis of restored teeth longitudinally cracked under cyclic loading. Dent Mater 2022;38(1):204-213.
- Ordinola-Zapata R, Lin F, Nagarkar S, Perdigão J. A critical analysis of research methods and experimental models to study the load capacity and clinical behaviour of the root filled teeth. Int Endod J 2022;55 Suppl 2(Suppl 2):471-494.
- Arola D, Reprogel RK. Effects of aging on the mechanical behavior of human dentin. Biomaterials 2005;26(18):4051-4061.
- Walton RE. Vertical root fracture: Factors related to identification. J Am Dent Assoc 2017;148(2):100-105.
- PradeepKumar AR, Shemesh H, Jothilatha S, Vijayabharathi R, Jayalakshmi S, Kishen A. Diagnosis of Vertical Root Fractures in Restored Endodontically Treated Teeth: A Time-dependent Retrospective Cohort Study. J Endod 2016;42(8):1175-1180.
- Yoshino K, Ito K, Kuroda M, Sugihara N. Prevalence of vertical root fractures as the reason for tooth extraction in dental clinics. Clinical Oral Investigations 2015;19:1405-1409.
- Stewart ML, McClanahan SB. Cemental tear: a case report. Int Endod J 2006;39(1):81-86.
- Pedercini A, Weitz DF, Heyse JD, Pedercini C, Kormas I, Koutlas IG, et al. Cemental tear: An overlooked finding associated with rapid periodontal destruction. A case series. Aust Dent J 2021;66 Suppl 1:S82-S87.
- Lin HJ, Chang MC, Chang SH, Wu CT, Tsai YL, Huang CC, et al. Treatment outcome of the teeth with cemental tears. J Endod 2014;40(9):1315-1320.
- Lin HJ, Chang SH, Chang MC, Tsai YL, Chiang CP, Chan CP, et al. Clinical fracture site, morphologic and histopathologic characteristics of cemental tear: role in endodontic lesions. J Endod 2012;38(8):1058-1062.
- Lee AHC, Neelakantan P, Dummer PMH, Zhang C. Cemental tear: Literature review, proposed classification and recommendations for treatment. Int Endod J 2021;54(11):2044-2073.
- Haney JM, Leknes KN, Lie T, Selvig KA, Wikesjö UM. Cemental tear related to rapid periodontal breakdown: a case report. J Periodontol 1992;63(3):220-224.
- Paul BF, Hutter JW. The endodontic-periodontal continuum revisited: new insights into etiology, diagnosis and treatment. J Am Dent Assoc 1997;128(11):1541-1548.
- Gupta S, Tewari S, Mittal S. Effect of Time Lapse between Endodontic and Periodontal Therapies on the Healing of Concurrent Endodontic-Periodontal Lesions without Communication: A Prospective Randomized Clinical Trial. J Endod 2015;41(6):785-790.
- Davis MC, Shariff SS. Success and Survival of Endodontically Treated Cracked Teeth with Radicular Extensions: A 2- to 4-year Prospective Cohort. J Endod 2019;45(7):848-855.
- Garrido I, Abella F, Ordinola-Zapata R, Duran-Sindreu F, Roig M. Combined Endodontic Therapy and Intentional Replantation for the Treatment of Palatogingival Groove. J Endod 2016;42(2):324-328.
Affiliations:
1 Division of Endodontics, Department of Restorative Sciences, School of Dentistry, University of Minnesota, Minneapolis.
2 Private Practice, Missoula, Montana.




