Bioceramic Usage in Endodontics
By Meetu R. Kohli, B.D.S., D.M.D., and Bekir Karabucak, D.M.D., M.S.
 Bioceramics refer to biomaterials that are used in direct contact with living tissue in the medical and dental field. Various types of bioceramics that are available in medicine and dentistry, include bioinert bioceramics like Zirconia and Alumina that do not react with the environment they are in contact with. Bioactive bioceramics that react with the tissue components and may either be bioresorbable like calcium phosphate bone substitute materials or non-bioresorbable such as the calcium silicate or hydraulic cements used in endodontics. Bioactivity of the materials refers to its ability to create a hydroxyapatite layer when in contact with tissue fluid rich in calcium and phosphate. This property allows the material to be highly biocompatible, osteoinductive, osteoconductive, and contributes to its sealing ability.
Bioceramics refer to biomaterials that are used in direct contact with living tissue in the medical and dental field. Various types of bioceramics that are available in medicine and dentistry, include bioinert bioceramics like Zirconia and Alumina that do not react with the environment they are in contact with. Bioactive bioceramics that react with the tissue components and may either be bioresorbable like calcium phosphate bone substitute materials or non-bioresorbable such as the calcium silicate or hydraulic cements used in endodontics. Bioactivity of the materials refers to its ability to create a hydroxyapatite layer when in contact with tissue fluid rich in calcium and phosphate. This property allows the material to be highly biocompatible, osteoinductive, osteoconductive, and contributes to its sealing ability.
The first-generation bioceramic or hydraulic cement introduced in endodontics is mineral trioxide aggregate (MTA) in the 1990s by Dr. M. Torabinejad. Hydraulic cements refer to a material that needs hydration to set and once set are impervious to dissolution in water. MTA is a medical grade mixture of Portland cement and bismuth oxide. Portland cement is primarily sintered trioxides of calcium, silica, and alumina. The manufacturing process leads to a mixture that consists of impurities, such as trace metals and metal oxides. Bismuth oxide is added to provide radioopacity for dental use. The material has endodontic applications from direct pulp cap to retrograde filling. It has been studied for three decades in various in-vitro, in-vivo and long term clinical prognostic data. Although the material is extremely biocompatible and has an excellent sealing ability it has some drawbacks. The new generation hydraulic cements have attempted to overcome these limitations.
One major limitation of first-generation bioceramic, MTA, has been the inability to provide as an endodontic sealer in its pure form. The coarse particle size of MTA that ranges from 1.5 – 160 microns does not allow it to be dispensed in a formulation that would flow adequately. Some formulations have been made available by adding resin and other additives to the material to allow for flowability. However, these additives are known to change the physical and biological properties of the material. New generation hydraulic cements use nanotechnology to reduce the particle size to a mixture of nano and microparticles and hence giving it the ability to flow. These are pure calcium silicate cements without any heavy metal oxides or other additives. The sealer sets with hydration reaction to produce calcium silicate hydrate and calcium hydroxide, which further reacts to form a hydroxyapatite layer in the presence of tissue fluid. The hydrophilic nature of the cement makes it an excellent choice to be used in the root canal system.
Introduction of new bioceramics has changed the use of these cements in endodontics. Traditionally, obturation of the root canal system involves maximizing the amount of gutta-percha and minimizing the layer of sealer whether used in warm vertical or cold lateral condensation. The aim is to limit the amount of sealer to the periphery of the gutta-percha to fill gaps and spaces and provide a seal. Resin-based sealer will shrink upon setting while calcium hydroxide and zinc oxide eugenol-based sealer can resorb over time hence a thin layer of sealer is preferred. In contrast, the silicate sealers expand while setting, the expansion is slight, less than 0.2% of total volume and once set will not resorb as easily. These properties of the sealer make it conducive to be used in larger volumes without the need to increase the volume of gutta-percha in the root canal, as it is a sealer based obturation. The purpose of the gutta-percha cone is to drive the sealer into cleaned isthmuses and irregular gaps. It also serves as a soft core that will allow for retreatment as set bioceramic cement is a challenge to go through with hand or rotary files.
Both white and grey MTA have shown to discolor remaining tooth structure over time, leading to esthetic failure of treatment. Bismuth oxide, traces of heavy metals and ferric oxide have been implicated in discoloration. The newer materials, such as Endosequence sealer/putty, BioRoot RCS (root canal sealer) powder/liquid, iRoot Sp and BioAggregate have eliminated bismuth oxide and heavy metals from its composition. These materials are primarily trisilicate cements and zirconia as the opacifier. The materials have shown less or no discoloration as compared to MTA in several investigations.
The newer bioceramics have been compared in in-vitro and animal studies to show similar or better results than mineral trioxide aggregate. Several clinical investigations in vital pulp therapy, perforation repair, and microsurgery show comparable or better results as well. As for sealer based obturation, one retrospective clinical investigation of 307 teeth with an average follow-up time of 30 months has an overall success rate of 90.9%. A higher level of evidence with randomized clinical trials is needed to prove the efficacy of the material long term. However, the hydrophilic nature, sealability, biocompatibility, antibacterial property, bioactivity, and ease of delivery has made it a promising material to be used in endodontics.
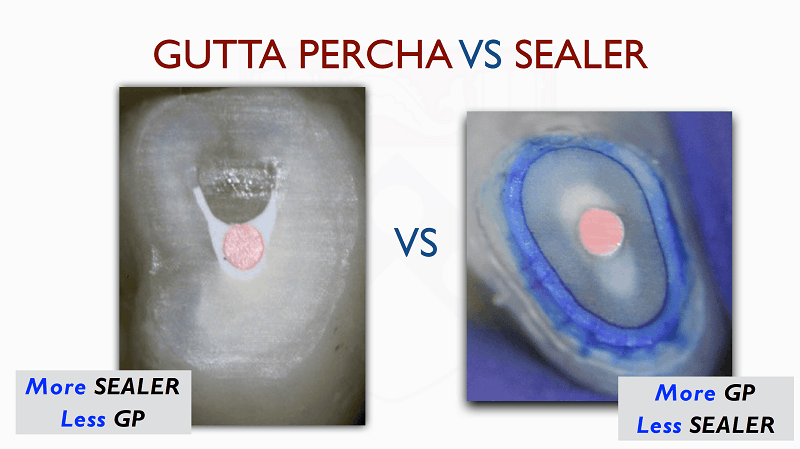
Figure: Sealer-based obturation with bioceramic sealer (left). Traditional obturation (right).
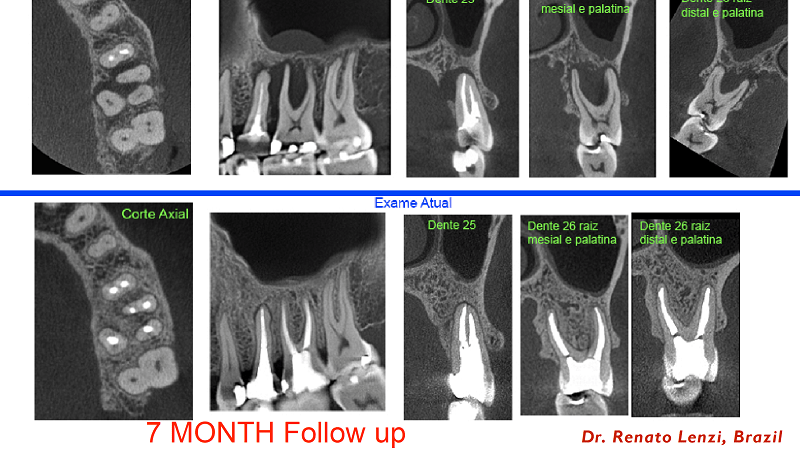
Case: Courtesy of Dr. Renato Lenzi, Brazil. Seven-month CBCT follow-up of #13 retreatment and #14 primary treatment with gutta percha and bioceramic sealer-based obturation. Preoperative CBCT (above row). Follow-up CBCT (row below).
Dr. Meetu R. Kohli, Diplomate, is clinical associate professor of endodontics and director, continuing education & International Scholar Program at University of Pennsylvania. Dr. Bekir Karabucak, Diplomate, is chair, associate professor, of endodontics, and director of the Postdoctoral Endodontics Program at University of Pennsylvania.
References cited:
Chen I, Karabucak B, Wang C, et al. Healing after root-end microsurgery by using mineral trioxide aggregate and a new calcium silicate-based bioceramic material as root-end filling materials in dogs. J Endod 2015;41:389–99.
Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review—part II: leakage and biocompatibility investigations. J Endod 2010;36: 190–202.
Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review—part III: clinical applications, drawbacks, and mechanism of action. J Endod 2010;36:400–13.
Trope M, Bunes A, Debelian G. Root filling materials and techniques: bioceramics a new hope? Endodontic Topics 2015, 32, 86–96
Mozynska J, Metlerski M, Lipski M, Nowicka A. Tooth Discoloration Induced by Different Calcium Silicate–based Cements: A Systematic Review of In Vitro Studies J Endod 2017: 43: 1593-1601
Almeida L, Moraes RR, Morgental RD, Pappen FG. Are Premixed Calcium Silicate–based Endodontic Sealers Comparable to Conventional Materials? A Systematic Review of In Vitro Studies. J Endod 2017;43:527-535
Shinbori N, Grama AM, Patel Y, Woodmansey K, He J. Clinical outcome of endodontic microsurgery that uses EndoSequence BC root repair material as the root-end filling material.J Endod. 2015 May;41(5):607-12..
Safi C, Kohli MR, Kratchman SI, Setzer FC, Karabucak B. Outcome of Endodontic Microsurgery Using Mineral Trioxide Aggregate or Root Repair Material as Root-end Filling Material: A Randomized Controlled Trial with Cone-beam Computed Tomographic Evaluation. J Endod. 2019 Jul;45(7):831-839.
Parirokh M, Torabinejad M, Dummer PMH. Mineral trioxide aggregate and other bioactive endodontic cements: an updated overview – part I: vital pulp therapy. Int Endod J. 2018 Feb;51(2):177-205.
Chybowski EA, Glickman GN, Patel Y, Fleury A, Solomon E, He J. Clinical Outcome of Non-Surgical Root Canal Treatment Using a Single-cone Technique with Endosequence Bioceramic Sealer: A Retrospective Analysis. J Endod. 2018 Jun;44(6):941-945.




